Pharmacological properties of the drug Marvelon
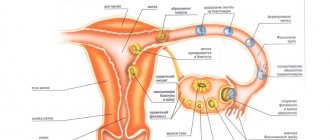
Pharmacodynamics. The action of combined oral contraceptives (COCs) is based on the interaction of various factors, the most important of which are suppression of ovulation and changes in cervical secretion. In addition to preventing pregnancy, PDAs have a number of positive properties that can be useful when choosing a contraceptive method. The menstrual cycle becomes more regular, periods are less painful, and blood loss is reduced. The latter may help reduce the incidence of iron deficiency anemia. There is evidence that the use of high-dose combined contraceptives (50 mcg ethinyl estradiol) reduces the risk of developing benign breast diseases, ovarian cysts, pelvic inflammatory diseases, ectopic pregnancy, endometrial and ovarian cancer. Pharmacokinetics. Desogestrel Absorption. Desogestrel taken orally is quickly and completely absorbed and converted to etonogestrel. Maximum plasma concentrations (about 2 ng/ml) are achieved approximately 1.5 hours after a single dose. Bioavailability is 62–81%. Distribution. Etonogestrel binds to serum albumin and sex hormone binding globulin (SHBG). Only 2–4% of the total plasma concentration is present as free steroid, and 40–70% is specifically bound to SHBG; Ethinyl estradiol-induced increase in SHBG content affects the distribution between blood plasma proteins, which leads to an increase in the SHBG-bound fraction and a decrease in the albumin-bound fraction; the expected volume of distribution of desogestrel is 1.5 l/kg. Metabolism. Etonogestrel is completely metabolized through known pathways of steroid metabolism; the rate of metabolic clearance from the blood is about 2 ml/min/kg body weight; no interaction with concomitantly administered ethinyl estradiol was detected. Excretion. The level of etonogestrel in the blood decreases biphasically; distribution in the final phase is characterized by a half-life of about 30 hours; desogestrel and its metabolites are excreted in urine and bile in a ratio of 6:4. The pharmacokinetics of etonogestrel is affected by the level of SHBG, which increases threefold under the influence of ethinyl estradiol; when taken daily, its serum level increases approximately 2–3 times, reaching a state of equilibrium in the second half of the course of drug use. Ethinyl estradiol Absorption. After oral administration, ethinyl estradiol is rapidly and completely absorbed; the maximum concentration in the blood serum (about 80 pg/ml) is achieved within 1–2 hours; absolute bioavailability as a result of first-pass conjugation and first-step metabolism is approximately 60%. Distribution. Ethinyl estradiol binds strongly, but not specifically, to serum albumin (approximately 98.5%) and causes an increase in serum concentrations of SHBG; volume of distribution - about 5 l/kg. Metabolism. Ethinyl estradiol is subject to presystemic conjugation both in the mucous membrane of the small intestine and in the liver; Ethinyl estradiol is first metabolized by aromatic hydroxylation, but this produces a large number of hydroxylated and methylated metabolites, which are subsequently present not only as free metabolites, but also as conjugates with glucuronides and sulfates; the rate of metabolic clearance is about 5 ml/min/kg. Excretion. The level of ethinyl estradiol in the blood decreases in two phases, the distribution in the final phase is characterized by a half-life of about 24 hours. The unchanged active substance is not excreted, ethinyl estradiol metabolites are excreted in the urine and bile in a ratio of 4:6; The half-life of metabolites is about 1 day. State of balance. Equilibrium concentrations are achieved after 3–4 days of taking the drug, when its level in the blood is 30–40% higher compared to taking a single dose.
Analogs
Analogues of the drug Marvelon:
- Regulon;
- Tri-Mercy;
- Mercilon;
- Novinet.
Each of these drugs contains a combination of desogestrel and ethinyl estradiol and, by and large, their main difference from each other is the price. Regulon as an alternative .
If the question arises, which is better - Marvelon or Regulon , you should pay attention to the fact that in terms of the content of active substances and pharmacological action, these drugs are absolutely identical. But the first of them is produced in the Netherlands, and the second in Russia, which in turn significantly affects the formation of prices for dispensing in pharmacy chains.
Use of the drug Marvelon
General rules for taking Marvelon Tablets should be taken daily for 21 days in accordance with the order indicated on the package without breaks, at approximately the same time, one tablet per day, with a small amount of liquid if necessary. After a 21-day course, take a break for 7 days. During this period, a menstrual-like reaction occurs, the duration of which may be shorter and the intensity lower than normal menstruation. As a rule, it begins on the 2-3rd day after taking the last tablet and can continue until the start of the next course of the drug (next package). Taking tablets from the next package begins after 7 days of the “pill-free” period. Starting to take Marvelon. Hormonal contraceptives were not used in the previous period (last month). Taking tablets begins on the 1st day of the natural cycle (that is, on the first day of menstrual bleeding). You can start taking it from days 2–5, but in this case, during the first cycle, it is recommended to additionally use a barrier method of contraception during the first 7 days of taking the pills. Switching from another PDA It is advisable that a woman start taking Marvelon the day after taking the last active pill of the previous combined contraceptive, but no later than the next day after a break in taking pills or placebo of the previous combined contraceptive. Switching from a method that is based on the use of progestogen only (mini-pills, injections, implants) A woman can start taking Marvelon on any day after stopping the use of the mini-pill (in the case of an implant - on the day of its removal, in the case of injections - instead next injection). However, in all cases it is recommended to additionally use a barrier method of contraception during the first 7 days of taking the pills. After an abortion in the first trimester, a woman can start taking Marvelon immediately. In this case, she does not need to use additional methods of contraception. After childbirth or abortion in the second trimester Women should be advised to start taking Marvelon on the 21st or 28th day after childbirth or abortion in the second trimester. If you start taking the pills later, you should additionally use a barrier method of contraception during the first 7 days of taking the pills. However, if sexual intercourse has already occurred, then before starting to use the PDA, it is necessary to establish the presence of pregnancy or wait until the next menstruation. What to do if you miss taking pills If the delay in taking the pill does not exceed 12 hours, the contraceptive effect of Marvelon will continue throughout the entire period of taking the drug. If for some reason a woman forgot to take a Marvelon tablet, it must be taken as soon as she finds out. In the future, taking tablets from this pack should continue as usual. If the delay in taking the pill exceeds 12 hours, the contraceptive effect may decrease. If you miss taking pills, you can follow 2 basic rules: • taking pills cannot be interrupted for more than 7 days; • to achieve an adequate inhibitory effect of the drug on the hypothalamus-pituitary-ovarian axis, continuous use of the drug is required for 7 days. In accordance with this, it is recommended to follow these tips. First week A woman should take the last missed pill as soon as she remembers the omission, even if she has to take 2 pills at the same time. She then continues to take the pills at the usual time. In addition, you must use a barrier method of contraception for the next 7 days. If you have had sexual intercourse within the previous 7 days, you should consider the possibility of pregnancy. The more pills you miss and the closer the missed pills are to your usual pill-free period, the higher the risk of pregnancy. Second week A woman should take the last missed pill as soon as she remembers, even if she has to take 2 pills at the same time. She then continues to take the pills at the usual time. If a woman has taken the pills correctly for 7 days before the missed pill, there is no need to use additional contraceptive measures. Otherwise, or if you miss more than 1 tablet, you should additionally use a barrier method of contraception for 7 days. Third week The risk of decreased contraceptive reliability increases as the break in taking pills approaches. However, if you follow the pill regimen, you can avoid a decrease in the level of contraception. If you adhere to one of the rules below, there will be no need to use additional contraceptives, provided that the woman took the pills correctly for 7 days before the missed pill. If not, then the woman should adhere to the first of the rules below and use additional methods of protection over the next 7 days. A woman should take the last missed pill as soon as she remembers, even if she has to take 2 pills at the same time. She then continues to take the pills at the usual time. Tablets from the next package should be started immediately after the previous one has ended, that is, there should be no break between taking tablets from different packages. It is unlikely that a woman will experience menstrual bleeding before finishing the second pack of tablets, although spotting or sudden bleeding may occur while taking the tablets. The woman may also be advised to stop taking the tablets in the current pack. In this case, the break should be 7 days, including days of missing pills; You should start taking the pills with the next pack. If a woman misses taking pills and does not have menstrual bleeding during the first regular break in taking the drug, pregnancy should be assumed. Recommendations in case of vomiting If vomiting occurs within 3-4 hours after taking the tablet, then incomplete absorption of the drug may occur. In this case, it is advisable to follow the recommendations regarding skipping pills. If a woman does not want to change her usual regimen of taking the drug, she needs to take an additional tablet from a different package. How to change or delay the onset of menstruation To delay the onset of menstruation, a woman should continue taking Marvelon from a new package and not take a break. If desired, the period of administration can be extended until the completion of taking the tablets from the second package. In this case, sudden short-term bleeding or spotting may occur. Regular use of Marvelon is resumed after the usual 7-day break from taking pills. In order to change the timing of menstruation - move its onset to another day of the week, a woman can be advised to shorten the period without taking pills by the required number of days. The shorter this period, the greater the likelihood of no withdrawal bleeding and the occurrence of spotting or short-term bleeding while taking the next package (as with a delay in menstruation).
Release form, composition and packaging
The tablets are white, round, biconvex, engraved with “TR” above the number “5” on one side and “ORGANON” with a five-pointed star on the other.
| 1 tab. | |
| ethinylestradiol | 30 mcg |
| desogestrel | 150 mcg |
Excipients: potato starch, povidone, stearic acid, colloidal silicon dioxide, α-tocopherol, lactose monohydrate.
21 pcs. - blisters (1) - foil sachets (1) - cardboard packs. 21 pcs. - blisters (1) - foil sachets (3) - cardboard packs. 21 pcs. - blisters (1) - foil sachets (6) - cardboard packs.
Contraindications to the use of the drug Marvelon
The use of combined contraceptives is contraindicated in any of the following conditions . If any of these conditions occur when using combined contraceptives for the first time, the drug should be stopped immediately:
- thrombosis (venous or arterial), including a history of indications (for example, deep vein thrombosis, pulmonary embolism, myocardial infarction, cerebrovascular disorders);
- the presence, including a history, of precursors of thrombosis (for example, angina pectoris, cerebrovascular accident);
- migraine with focal neurological symptoms;
- diabetes mellitus with vascular complications;
- presence of thrombosis;
- pancreatitis or previous pancreatitis, accompanied by high hypertriglyceridemia;
- severe liver diseases, including the presence of indications in the anamnesis (until normalization of liver function indicators);
- liver tumors (benign or malignant), including history;
- hormone-dependent malignant tumors (established or suspected, for example tumors of the genital organs or mammary glands);
- vaginal bleeding of unknown etiology;
- established or suspected pregnancy;
- hypersensitivity to any of the components of the drug.
COC products should be taken with caution in any of the following conditions: obesity (body mass index 30 kg/m2);
increased blood pressure; atrial fibrillation; heart valve disease; dyslipoproteinemia; liver and gallbladder disease; Crohn's disease and ulcerative colitis; sickle cell anemia; systemic lupus erythematosus; hemolytic uremic syndrome; epilepsy; smoking over the age of 35; prolonged immobilization, significant surgical interventions; fibrocystic mastopathy; uterine fibroids; diabetes; congenital hyperbilirubinemia (Gilbert, Dubin-Johnson and Rotor syndrome); chloasma (women predisposed to pigmentation are advised to avoid exposure to the sun or ultraviolet radiation while taking COCs).
Special instructions for the use of the drug Marvelon
The daily dose of lactose contained in the tablet is so low (≤80 mg) that even lactose-sensitive women will not experience any disturbances. Caution If any of the conditions/risk factors listed in the CONTRAINDICATIONS section are present, the benefits and risks of using COCs should be considered by a physician individually for each woman and discussed with the patient before she decides to take this drug. In case of exacerbation, intensification or the first appearance of any of these conditions, a woman should consult a doctor. Your doctor must determine whether you should continue or stop taking the COC. Medical examination/consultation Before starting or resuming oral use of combined contraceptives, a thorough medical examination of the woman should be performed, including obtaining a full medical history and performing instrumental studies, paying particular attention to possible contraindications. Such an examination must be repeated at least once a year during the entire period of taking combined contraceptives. Periodic medical evaluation is important regarding contraindications (for example, cerebrovascular accidents) or risk factors (for example, a family history of venous or arterial thrombosis) that may first appear while taking contraceptives. The frequency and nature of such examinations are determined for each woman individually, but in general they should include blood pressure measurement, examination of the mammary glands, abdominal and pelvic organs, cytological examination of the cervical epithelium and relevant laboratory tests. It is necessary to explain to women that oral contraceptives do not protect against HIV/AIDS and other sexually transmitted diseases. Decreased effectiveness The effectiveness of COCs may be reduced if tablets are missed (see APPLICATION), gastrointestinal disorders, or if certain medications are taken at the same time (see INTERACTIONS). Deterioration of control of the menstrual cycle While using COCs, irregular minor (spotting) or heavy bleeding may occur, especially during the first months of use. Therefore, assessment of any disturbances in menstrual cycle control can only be made after an adaptation period of approximately 3 cycles. If irregular bleeding persists or occurs after previous regular cycles, probable non-hormonal causes should be considered and the necessary diagnostic tests should be performed to exclude pregnancy or a tumor; curettage is possible. Some women may not experience withdrawal bleeding during the pill-free interval. If PDAs were used according to recommendations, then the possibility of pregnancy is low. However, if there has been a violation of these recommendations in the period before the first absence of bleeding during the period without taking pills, or if there is no bleeding twice in a row, the possibility of pregnancy should be excluded before continuing to take the COC. Use of the drug during pregnancy and/or breastfeeding. Pregnancy is a contraindication for the use of Marvelon. If a woman becomes pregnant while taking Marvelon, further use should be stopped immediately. Studies have not revealed an increased risk of pathology in children born to mothers who took COCs during pregnancy, nor a teratogenic effect from COCs that were unintentionally taken early in pregnancy. Lactation is influenced by estrogens because they can reduce the quantity and change the composition of breast milk, but there has been no evidence of a negative effect on the health of infants. However, it is not recommended to use COCs during breastfeeding; if you need to use COCs, you should completely stop breastfeeding. Impact on the ability to drive vehicles. Does not affect the ability to concentrate.
Side effects
In some cases, while taking Marvelon, some women may experience side effects, which are expressed in the form of dysfunction of individual systems and organs.
So, from the side of the reproductive system the following are not excluded:
- intermenstrual bleeding from the genital tract ;
- violation of cervical secretion;
- hardening and swelling of the mammary glands ;
- amenorrhea.
The most likely side effects that occur from the gastrointestinal tract are attacks of nausea and vomiting .
The central nervous system may respond to the drug with symptoms such as dizziness , mood lability, headaches or migraines .
In some cases, fluid retention in the body and fluctuations in body weight may occur. Also possible:
- the appearance of chloasma ;
- the appearance erythema nodosum ;
- the appearance of skin rashes;
- decreased tolerance or complete tolerance of contact lenses;
- increased risk of developing acute blockage of a blood vessel by a thrombus.
Interactions of the drug Marvelon
Interactions between oral contraceptives and other drugs may lead to sudden bleeding and/or a decrease in the contraceptive effect of the drug. Interactions may occur with drugs that induce microsomal enzymes, which can lead to increased metabolism of sex hormones. These drugs include phenytoin, barbiturates, primidone, carbamazepine, rifampicin and possibly also oxcarbazepine, topiramate, felbamate, ritonavir, griseofulvin and preparations containing St. John's wort. The effectiveness of the drug may also be reduced if certain antibiotics are taken concomitantly, such as penicillins and tetracyclines. These drugs reduce the enterohepatic circulation of estrogens, which leads to a decrease in ethinyl estradiol concentrations. Women taking any of these drugs should temporarily use a barrier method of contraception in addition to POCs or choose another method to protect against unwanted pregnancy. Women taking antibiotics (except rifampicin and griseofulvin) need to use a barrier method while taking the corresponding drug and for 7 days after its discontinuation. When taking drugs that stimulate microsomal enzymes, it is necessary to use a barrier method in addition to COCs throughout the entire period of treatment and for another 28 days after stopping treatment. If the administration of the drug must be continued, and the tablets in the CCP pack have already run out, the next pack should be started immediately, without the usual break. COCs may affect the metabolism of other drugs. Accordingly, the concentration of such drugs (for example, cyclosporine) in the blood plasma and tissues may change. Note. To determine possible interactions, information regarding the prescription of the drug that is used in parallel should be reviewed. Laboratory tests. Contraceptive steroids may affect the results of certain laboratory tests, including biochemical indicators of liver, kidney, thyroid, adrenal function, serum levels of proteins (transporters), such as corticosteroid binding globulin and/or lipid/lipoprotein fractions, indicators of carbohydrate metabolism, coagulation and fibrinolysis. Changes usually remain within the normal range.
Overdose
Both desogestrel and ethinyl estradiol are low-toxic substances, so the likelihood of symptoms threatening a woman’s health if they overdose is assessed as extremely low.
If you accidentally take several Marvelon tablets at the same time, in some cases the following may occur:
- vomit;
- nausea;
- slight bloody vaginal discharge (in young patients).
antidotes to the drug. If signs of overdose appear, the patient is prescribed symptomatic treatment .