16862
Author of the article
Evgeniy Nikolaevich Konoplev
Reading time: 5 minutes
AA
Baklosan tablets are a new generation of muscle relaxants that are used to relieve increased muscle tone and eliminate pain due to muscle spasms. The drug is recommended for the complex treatment of multiple sclerosis, pathologies of the musculoskeletal system, and cerebral stroke. The medicine helps eliminate cramps in muscle tissue, facilitate massage and physical therapy.
Pharmacodynamics and pharmacokinetics
Pharmacodynamics
Baklosan is a centrally acting muscle relaxant GABAB receptor inhibitor . Reduces the excitability of the distal parts of the sensory fibers carrying nerve impulses and inhibits the intermediate cells of the nervous system. As a result, synaptic transmission of impulses is inhibited; reduction in muscle fiber tension. The drug does not affect the transmission of nerve impulses at muscle-nervous synapses.
For diseases characterized by pathological constant contractility of skeletal muscles, the drug Baclosan relieves cramps and painful spasms. The drug increases the range of motion in the joints, simplifies kinesiotherapy (massage, gymnastics, manual therapy).
Pharmacokinetics
After taking Baklosan tablets, they are highly adsorbed in the intestines. The maximum concentration in the blood is observed three hours after administration of the drug.
Plasma protein binding reaches 30%. The drug penetrates the placental barrier and is excreted in breast milk. Metabolism occurs in the liver. Excreted in urine in its original form.
Additionally
Side effects are often transient in nature, they should be distinguished from the symptoms of the disease for which treatment is being carried out: with concomitant epilepsy, treatment is carried out without discontinuation of antiepileptic drugs.
In patients with liver disease and diabetes mellitus, it is necessary to periodically monitor the activity of liver transaminases, alkaline phosphatase, and blood glucose concentration.
During treatment, you should refrain from potentially dangerous activities associated with the need for increased attention and rapid mental and motor reactions.
Contraindications
Contraindications to the use of Baklosan are:
- Parkinson's disease;
- psychoses;
- seizures (including in the past);
- epilepsy;
- lactose intolerance lactase deficiency , malabsorption ;
- stomach or duodenal ulcer;
- pregnancy;
- lactation period;
- allergy to the components of the drug.
Baklosan is used with caution in cases of insufficient blood supply to the brain, atherosclerosis of cerebral vessels, renal failure , in the elderly and children under three years of age.
Pharmacological group
| Category ICD-10 | Synonyms of diseases according to ICD-10 |
| C72.0 Spinal cord | Spinal cord tumors |
| D43.4 Spinal cord | Spinal cord tumors |
| G03.9 Meningitis, unspecified | Arachnoiditis |
| Subacute meningitis | |
| Consequence of meningitis | |
| Serous meningitis | |
| Cerebral meningitis | |
| G04 Encephalitis, myelitis and encephalomyelitis | Inflammation of the meninges |
| Disseminated acute encephalomyelitis | |
| Leukoencephalitis | |
| Meningomyelitis | |
| Myelitis | |
| Acute encephalitis | |
| Acute encephalomyelitis | |
| Chronic encephalitis | |
| Chronic encephalomyelitis | |
| Encephalitis | |
| Encephalomyelitis | |
| G12.2 Motor neuron disease | Amyotrophic lateral sclerosis |
| Amyotrophic lateral sclerosis | |
| Motor neuron diseases | |
| Lowe-Gehrig's disease | |
| Bulbar palsies | |
| Bulbar palsy | |
| Primary lateral sclerosis | |
| Progressive bulbar palsy | |
| Progressive pseudobulbar palsy | |
| Amyotrophic lateral sclerosis | |
| Amyotrophic lateral sclerosis | |
| Primary lateral sclerosis | |
| Tropical spastic paraparesis | |
| Charcot's disease | |
| G35 Multiple sclerosis | Secondary progressive multiple sclerosis |
| Disseminated sclerosis | |
| Multiple sclerosis | |
| Exacerbation of multiple sclerosis | |
| Relapsing multiple sclerosis | |
| Mixed forms of multiple sclerosis | |
| G80 Cerebral palsy | Infantile cerebral palsy |
| Cerebral paralysis | |
| G95 Other diseases of the spinal cord | Spinal cord diseases |
| G95.0 Syringomyelia and syringobulbia | Syringomyelia |
| I67.9 Cerebrovascular disease, unspecified | Angioneuropathy |
| Arterial angiopathy | |
| Brain hypoxia | |
| Encephalopathy | |
| Diseases of the brain of a vascular and age-related nature | |
| Coma due to cerebrovascular accident | |
| Lacunar status | |
| Metabolic and vascular disorders of the brain | |
| Impaired blood supply to the brain | |
| Cerebrovascular accident | |
| Brain dysfunction | |
| Dysfunction of the cerebral cortex | |
| Cerebral circulation disorders | |
| Cerebrovascular insufficiency | |
| Acute cerebrovascular insufficiency | |
| Acute cerebrovascular accident | |
| Damage to cerebral vessels | |
| Progression of destructive changes in the brain | |
| Cerebrovascular disorders | |
| Cerebral insufficiency syndrome | |
| Cerebrovascular insufficiency | |
| Vascular encephalopathy | |
| Vascular diseases of the brain | |
| Vascular disorders of the brain | |
| Vascular lesions of the brain | |
| Functional brain disorders | |
| Chronic cerebral ischemia | |
| Chronic circulatory failure | |
| Chronic cerebrovascular insufficiency | |
| Chronic cerebrovascular insufficiency | |
| Chronic disorder of blood supply to the brain | |
| Cerebral insufficiency | |
| Cerebral organic failure | |
| Cerebrostenia | |
| Cerebrovascular pathology | |
| Cerebroasthenic syndrome | |
| Cerebrovascular disease | |
| Cerebrovascular disease | |
| Cerebrovascular disorder | |
| Cerebrovascular disorder | |
| Discirculatory encephalopathy | |
| S06 Intracranial injury | Brain injury |
| Brain injuries | |
| Traumatic brain damage | |
| Consequences of a TBI | |
| Consequence of traumatic brain injury | |
| Consequence of TBI | |
| Condition after traumatic brain injury | |
| Concussion | |
| Traumatic brain injury | |
| Traumatic cerebrovascular disease | |
| Traumatic brain injury | |
| Traumatic brain lesions | |
| Brain injuries | |
| Skull injuries | |
| Brain contusion | |
| Traumatic Trauma | |
| Traumatic brain injury | |
| Traumatic brain injury with predominantly brain stem level of damage | |
| TBI | |
| T09.3 Spinal cord injury at unspecified level | Spinal injury |
| Traumatic spinal cord injury |
Side effects
- From the musculoskeletal system: myalgia .
- From the nervous system: weakness, dizziness, gait disturbance, insomnia, sedation , depression , euphoria , asthenia , tremor , hallucinations, nightmares, nystagmus , paresthesia , dysarthria .
- From the respiratory system: respiratory depression.
- From the cardiovascular system: decreased cardiac output , hypotension .
- From the organs of vision: visual disturbances.
- From the digestive system: diarrhea , constipation , dyspepsia , nausea, abdominal pain, liver damage.
- From the genitourinary system: enuresis , polyuria , urinary retention, and sometimes erectile dysfunction .
- Skin: hyperhidrosis .
- General disorders: decreased temperature.
In extremely rare cases, an increase in spasms was detected, which was assessed as a paradoxical effect of taking the drug.
Indications for use
It is recommended that people take this drug only after a full examination by a doctor.
Indications for use:
- suffered a stroke;
- muscle hypertonicity for patients with multiple sclerosis;
- cerebral palsy;
- meningitis;
- state of affect and nervous system disorder in alcohol-dependent people;
- previous injuries;
- consequences of previous infectious pathology;
- process of degeneration in the body.
Instructions for use of Baklosan (Method and dosage)
Instructions for use of Baklosan recommend taking the drug orally with food. If one dose of the drug was missed by mistake, the next time you should not take a double dose.
Adults
The starting dose is 5 mg (half a 10 mg tablet) three times a day, increasing the dose by 5 mg every three days and so on until the therapeutic effect occurs (usually 30-75 mg per day is sufficient). The maximum daily dose is 100 mg.
The final dose should not decrease muscle tone, cause significant myasthenia , or impair motor function.
For renal failure or during hemodialysis , the daily dose is 5 mg.
In persons over 65 years of age, the dose should be increased with caution, as they are at high risk of developing side effects.
Children
The starting dose is 5 mg three times a day. If necessary, the dose can be increased every three days by 5 mg until a therapeutic effect appears.
Recommended doses: for children aged 3-6 years – 20-30 mg/day; 6-10 years – 30-60 mg/day; over 10 years of age, the maximum daily dose is 2.5 mg/kg of body weight, the starting dose is 1.5-2 mg/kg of body weight.
The drug is withdrawn slowly (within 10-14 days).
Synonyms of nosological groups
| Category ICD-10 | Synonyms of diseases according to ICD-10 |
| C72.0 Spinal cord | Spinal cord tumors |
| D43.4 Spinal cord | Spinal cord tumors |
| G03.9 Meningitis, unspecified | Arachnoiditis |
| Subacute meningitis | |
| Consequence of meningitis | |
| Serous meningitis | |
| Cerebral meningitis | |
| G04 Encephalitis, myelitis and encephalomyelitis | Inflammation of the meninges |
| Disseminated acute encephalomyelitis | |
| Leukoencephalitis | |
| Meningomyelitis | |
| Myelitis | |
| Acute encephalitis | |
| Acute encephalomyelitis | |
| Chronic encephalitis | |
| Chronic encephalomyelitis | |
| Encephalitis | |
| Encephalomyelitis | |
| G12.2 Motor neuron disease | Amyotrophic lateral sclerosis |
| Amyotrophic lateral sclerosis | |
| Motor neuron diseases | |
| Lowe-Gehrig's disease | |
| Bulbar palsies | |
| Bulbar palsy | |
| Primary lateral sclerosis | |
| Progressive bulbar palsy | |
| Progressive pseudobulbar palsy | |
| Amyotrophic lateral sclerosis | |
| Amyotrophic lateral sclerosis | |
| Primary lateral sclerosis | |
| Tropical spastic paraparesis | |
| Charcot's disease | |
| G35 Multiple sclerosis | Secondary progressive multiple sclerosis |
| Disseminated sclerosis | |
| Multiple sclerosis | |
| Exacerbation of multiple sclerosis | |
| Relapsing multiple sclerosis | |
| Mixed forms of multiple sclerosis | |
| G80 Cerebral palsy | Infantile cerebral palsy |
| Cerebral paralysis | |
| G95 Other diseases of the spinal cord | Spinal cord diseases |
| G95.0 Syringomyelia and syringobulbia | Syringomyelia |
| I67.9 Cerebrovascular disease, unspecified | Angioneuropathy |
| Arterial angiopathy | |
| Brain hypoxia | |
| Encephalopathy | |
| Diseases of the brain of a vascular and age-related nature | |
| Coma due to cerebrovascular accident | |
| Lacunar status | |
| Metabolic and vascular disorders of the brain | |
| Impaired blood supply to the brain | |
| Cerebrovascular accident | |
| Brain dysfunction | |
| Dysfunction of the cerebral cortex | |
| Cerebral circulation disorders | |
| Cerebrovascular insufficiency | |
| Acute cerebrovascular insufficiency | |
| Acute cerebrovascular accident | |
| Damage to cerebral vessels | |
| Progression of destructive changes in the brain | |
| Cerebrovascular disorders | |
| Cerebral insufficiency syndrome | |
| Cerebrovascular insufficiency | |
| Vascular encephalopathy | |
| Vascular diseases of the brain | |
| Vascular disorders of the brain | |
| Vascular lesions of the brain | |
| Functional brain disorders | |
| Chronic cerebral ischemia | |
| Chronic circulatory failure | |
| Chronic cerebrovascular insufficiency | |
| Chronic cerebrovascular insufficiency | |
| Chronic disorder of blood supply to the brain | |
| Cerebral insufficiency | |
| Cerebral organic failure | |
| Cerebrostenia | |
| Cerebrovascular pathology | |
| Cerebroasthenic syndrome | |
| Cerebrovascular disease | |
| Cerebrovascular disease | |
| Cerebrovascular disorder | |
| Cerebrovascular disorder | |
| Discirculatory encephalopathy | |
| S06 Intracranial injury | Brain injury |
| Brain injuries | |
| Traumatic brain damage | |
| Consequences of a TBI | |
| Consequence of traumatic brain injury | |
| Consequence of TBI | |
| Condition after traumatic brain injury | |
| Concussion | |
| Traumatic brain injury | |
| Traumatic cerebrovascular disease | |
| Traumatic brain injury | |
| Traumatic brain lesions | |
| Brain injuries | |
| Skull injuries | |
| Brain contusion | |
| Traumatic Trauma | |
| Traumatic brain injury | |
| Traumatic brain injury with predominantly brain stem level of damage | |
| TBI | |
| T09.3 Spinal cord injury at unspecified level | Spinal injury |
| Traumatic spinal cord injury |
Baklosan tablets must be stored in a dark and dry place, out of reach of children, at a temperature below 25º.
Interaction
When used together, Baklosan activates the action of drugs that affect the nervous system, lower blood pressure, anti-gout drugs, and alcohol.
When Baklosan is taken simultaneously with tricyclic antidepressants, a pronounced decrease in muscle tone develops.
You should not take Baclosan with carbidopa and levodopa , as confusion and hallucinations may occur.
Storage, release from pharmacies
Baklosan tablets are stored at room temperature up to 25 degrees . They should be placed in a dry, dark place where children cannot reach. The medicine is released only by prescription , as it is a pharmaceutical drug.
Shelf life is 5 years, but this period may be reduced due to improper storage.
Baklosan's analogs
Level 4 ATC code matches:
Tizanil
Tizanidine
Tolperisone-OBL
Tizalud
Mydocalm
Sirdalud
Baclofen
Lioresal and Baclofen are the main analogues of Baclosan.
Help with poisoning
There is no special antidote for an overdose of Baklosan. If one or more symptoms of poisoning occur, the first step is to stop taking the medication and get rid of the remaining drug in the body. Baclofen affects the spasmodic contractions of the stomach muscles and the removal of the drug from the body slows down. However, gastric lavage should be continued.
You can let the patient drink 2 liters of salted water and continue to induce a gag reflex by pressing your finger on the root of the tongue, after which you need to replenish the fluid lost by the body. Enterosorbents are used to remove toxic substances. Inpatients at the hospital use hydration therapy (Ringer's solution and sodium chloride) with diuretics to reduce the dose of the drug in the body.
To stabilize the dropped blood pressure and the activity of the heart muscles, the patient is administered adrenaline or glucocorticoid hormones and respiratory activity is monitored. In some cases, artificial ventilation is used. Treatment in a hospital can last a week or even longer, depending on the severity of the patient’s condition.
As a rule, Baklosan is well tolerated. To avoid overdose and drug poisoning, you must follow your doctor’s recommendations and not self-medicate.
Reviews about Baklosan
Recommendations and reviews of Baklosan indicate that most patients were satisfied with the results of using the drug. It is rare to read negative reviews about Baklosan tablets associated with side effects.
A number of patients in drug treatment clinics often use the drug in combination with certain drugs. In this case, baklosan is capable of causing a trip or “high.” It is known that such use of the drug results in the development of addiction.
How to “get off” the drug Baklosan?
This is a question often asked by patients. If withdrawal syndrome occurs as a result of using the drug in recommended doses, then discontinuation of use should be done gradually over two weeks. If addiction has arisen as a result of unauthorized use of the drug to achieve a narcotic effect, then you need to seek help from a narcologist.
How to get rid of addiction
The drug does not cause chemical addiction; it is predominantly psychological, and traditional drug treatment in this case is ineffective. The peculiarity of baklosan is that it is necessary to control its withdrawal, monitor the patient’s condition, gradually returning him to a full, healthy life. The Narconon program provides this effect.
The technique does not involve drug detoxification (this is important in the treatment of psychological addictions). The consequences of damage to the body are eliminated by taking vitamin-mineral complexes, niacin, and oil mixtures. They are supplemented by diet, running, and visiting the sauna. The physical and psychological consequences of intoxication during drug withdrawal are eliminated with the help of assistive procedures performed by specially trained staff.
Healthy communication skills are returned by sequentially going through the following stages:
- “Objective processes” - gaining a clear view of what is happening;
- “Overcoming ups and downs in life” - support aimed at avoiding temptations, harmful, old social circles;
- “Personal values” - liberation from the past and awareness of one’s own responsibility for actions;
- “Changing states in life” - developing the ability to creatively solve problems;
- “Life Skills” - obtaining tools for conscious and complete abstinence from drugs.
On average, the program lasts from 8 to 10 weeks. The result of its complete completion is cessation of drug use and preservation of the effect for life.
Share:
Trigan D is a common drug among teenagers.
Diazepam
Baklosan price, where to buy
The price of Baklosan in Russia 10 mg is 213-256 rubles, buying 50 tablets of 25 mg will cost 405-498 rubles. In Ukraine, the average price of Baklosan tablets 10 mg is 59 hryvnia, 25 mg is 104 hryvnia.
- Online pharmacies in RussiaRussia
ZdravCity
- Baklosan tablets 10 mg 50 pcs.Polpharma
252 rub.order
Release forms
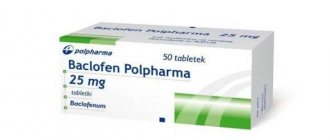
There are 2 release forms: tablets with 25 and 10 mg of the active ingredient baclofen. Packed in 50 pcs. in polypropylene jars or cardboard packs.
Tablets 25mg
25 mg tablets are round, convex on both sides, white. They do not have a dividing strip. Sold for 480-500 rubles per pack of 50 pcs.
Tablets 10mg
The 10 mg tablets are similar to the 25 mg tablets but contain a release strip. Sold for 250-275 rubles per 50 pcs.