Brief information about the medicine
The blood pressure medication Dalneva is a combination drug that belongs to the pharmacological group of ACE inhibitors and calcium blockers. Produced in Russia.
Release form
Blood pressure medicine Dalneva is produced by the manufacturer in tablet form. The tablets are packaged in blisters - 10 pieces each. Sold in cardboard packages containing 3 or 6 blisters.
Compound
The main active components of the drug are perindopril and amlodipine, at a dosage of 8 and 10 mg, respectively. The composition of the tablets also includes such auxiliary ingredients as magnesium stearate, microcrystalline cellulose, sodium bicarbonate, starch, anhydrous colloidal silicon dioxide.
Packaging of Dalneva tablets
pharmachologic effect
Dalneva's remedy for high blood pressure is characterized by the presence of pronounced antianginal and hypotensive properties.
Terms and conditions of storage
The maximum permissible shelf life of the drug is 3 years. The tablets should be stored out of the reach of small children and animals, at a temperature not exceeding +15-20°C.
Terms of sale
You can purchase a medicine in pharmacy chains only upon presentation of an expired medical prescription.
Price
The exact cost of the drug depends on the number of tablets purchased. The average price of Dalnev tablets varies from 400 to 600 rubles.
Dalneva
Special instructions related to amlodipine and perindopril also apply to Dalneva.
Perindopril
Hypersensitivity/angioedema (Quincke's edema)
When using ACE inhibitors, including perindopril, in rare cases, the development of angioedema of the face, lips, tongue, vocal folds, and/or larynx may occur. If these symptoms appear, use of the drug should be stopped immediately, and the patient should be observed until signs of edema disappear completely.
If angioedema affects only the face and lips, its symptoms usually resolve on their own, or antihistamines can be used to treat the symptoms. Angioedema, accompanied by swelling of the tongue or larynx, can lead to airway obstruction and death.
If such symptoms appear, you should immediately administer epinephrine (adrenaline) subcutaneously at a dilution of 1:1000 (0.3 or 0.5 ml) and/or ensure airway patency. The patient should be under medical supervision until symptoms disappear completely and permanently.
Patients with a history of angioedema not associated with the use of ACE inhibitors may have an increased risk of developing it when using drugs in this group.
In rare cases, intestinal angioedema (angioedema of the intestine) develops during therapy with ACE inhibitors. In this case, patients experience abdominal pain as an isolated symptom or in combination with nausea and vomiting, in some cases without previous angioedema of the face and with normal C-1-esterase levels. The diagnosis is made using computed tomography of the abdominal cavity, ultrasound, or at the time of surgery. Symptoms disappear after stopping the use of ACE inhibitors. In patients with abdominal pain receiving ACE inhibitors, the possibility of developing intestinal angioedema must be taken into account when making a differential diagnosis.
Anaphylactoid reactions during desensitization procedures
There are isolated reports of prolonged, life-threatening anaphylactoid reactions in patients receiving ACE inhibitors during desensitization therapy with Hymenoptera venom. ACE inhibitors should be used with caution in patients prone to allergic reactions undergoing desensitization procedures. Prescription of an ACE inhibitor should be avoided in patients receiving immunotherapy with hymenoptera venom. However, the development of anaphylactoid reactions can be avoided by temporarily discontinuing the ACE inhibitor at least 24 hours before the start of the desensitization procedure.
Anaphylactoid reactions during LDL apheresis using dextran sulfate
In rare cases, life-threatening anaphylactoid reactions may occur in patients receiving ACE inhibitors during low-density lipoprotein (LDL) apheresis using dextran sulfate. To prevent an anaphylactoid reaction, ACE inhibitor therapy should be discontinued before each LDL apheresis procedure using high-flux membranes.
Hemodialysis
Anaphylactic reactions have been observed in patients receiving ACE inhibitors during hemodialysis using high-flow membranes. Therefore, it is advisable to use a different type of membrane or use an antihypertensive drug of a different pharmacotherapeutic group.
Neutropenia/agranulocytosis, thrombocytopenia and anemia
In patients taking ACE inhibitors, cases of neutropenia/agranulocytosis, thrombocytopenia and anemia may develop. In patients with normal renal function in the absence of other complications, neutropenia rarely develops and resolves spontaneously after discontinuation of ACE inhibitors.
Perindopril should be used with great caution in patients with connective tissue diseases and simultaneously receiving immunosuppressive therapy, allopurinol or procainamide, especially with existing renal impairment. Some patients may develop severe infections that do not respond to intensive antibiotic therapy. If perindopril is prescribed, monitoring the number of leukocytes in the blood plasma is recommended. The patient should be warned that if any signs of an infectious disease appear (sore throat, fever), consult a doctor immediately.
Risk of arterial hypotension and/or renal failure (in patients with chronic heart failure, fluid and electrolyte imbalance, etc.)
In liver cirrhosis, accompanied by edema and ascites, arterial hypotension, and CHF, significant activation of the renin-angiotensin-aldosterone system (RAAS) may be observed, especially with severe hypovolemia and a decrease in the content of electrolytes in the blood plasma (against the background of a diet with limited salt or long-term use of diuretics ).
The use of an ACE inhibitor causes blockade of the RAAS, and therefore a sharp decrease in blood pressure and/or an increase in the concentration of creatinine in the blood plasma is possible, indicating the development of acute renal failure, which is more often observed when taking the first dose or during the first two weeks of therapy.
ACE inhibitors can cause a sharp decrease in blood pressure. Symptomatic hypotension rarely occurs in patients without underlying medical conditions. The risk of an excessive decrease in blood pressure is increased in patients with reduced blood volume, which can be observed during diuretic therapy, while following a strict diet with limited salt, hemodialysis, with diarrhea or vomiting, or in patients with severe arterial hypertension with high renin activity. In patients at high risk of developing symptomatic hypotension, blood pressure, renal function, and serum potassium levels should be carefully monitored during drug therapy.
The same precautions apply to patients with angina pectoris or cerebrovascular diseases, in whom a pronounced decrease in blood pressure can lead to the development of myocardial infarction or cerebrovascular accident.
If arterial hypotension develops, the patient should be transferred to the supine position with legs elevated. If necessary, replenish the blood volume with intravenous administration of 0.9% sodium chloride solution. Transient arterial hypotension is not a contraindication for further use of the drug. After restoration of blood volume and blood pressure, therapy can be continued.
Aortic stenosis/Mitral stenosis/Hypertrophic obstructive cardiomyopathy
ACE inhibitors should be used with caution in patients with left ventricular outflow tract obstruction (aortic stenosis, hypertrophic obstructive cardiomyopathy), as well as in patients with mitral stenosis.
Potassium-sparing diuretics and potassium supplements
The simultaneous use of perindopril and potassium-sparing diuretics, as well as potassium preparations and potassium-containing salt substitutes is not recommended.
Cough
During therapy with an ACE inhibitor, a dry, nonproductive cough may occur, which disappears after discontinuation of drugs in this group. If a dry cough appears, you should be aware of the possible connection of this symptom with the use of an ACE inhibitor.
Children and adolescents under 18 years of age
The drug is contraindicated in children and adolescents under 18 years of age due to the lack of data on the effectiveness and safety of the drug in this age group.
Renal dysfunction
If renal function is impaired (creatinine clearance less than 60 ml/min), individual selection of doses of perindopril and amlodipine is recommended. Regular monitoring of potassium and creatinine levels in the blood plasma is a necessary condition in the treatment of such patients.
In some patients with bilateral renal artery stenosis or stenosis of the artery of a solitary kidney, taking ACE inhibitors, there was an increase in plasma urea and creatinine concentrations, reversible after discontinuation of therapy. These changes are more likely in patients with renal failure. Patients with renovascular hypertension are at increased risk of severe hypotension and renal failure. In some hypertensive patients without obvious evidence of existing renal disease who took perindopril concomitantly with a diuretic, small and transient increases in serum urea and creatinine concentrations were observed. These changes more often develop in patients with pre-existing renal impairment.
Liver dysfunction
Rarely, the use of ACE inhibitors is accompanied by a syndrome, the development of which begins with cholestatic jaundice and which then progresses to fulminant liver necrosis, sometimes with death. The mechanism of development of this syndrome is unclear. If jaundice occurs or liver transaminases increase during use of an ACE inhibitor, the ACE inhibitor should be discontinued immediately and the patient should remain under appropriate medical supervision.
Ethnic characteristics
In patients of the Negroid race, angioedema develops more often than in representatives of other races while using ACE inhibitors. Perindopril, like other ACE inhibitors, may have a less pronounced hypotensive effect in patients of the Black race compared to representatives of other races. Perhaps this difference is due to the fact that patients with arterial hypertension of the Negroid race more often have low plasma renin activity.
Surgery/general anesthesia
The use of ACE inhibitors in patients undergoing major surgery and/or general anesthesia can lead to a significant decrease in blood pressure if general anesthesia agents with a hypotensive effect are used. This is due to blocking the formation of angiotensin II against the background of a compensatory increase in renin activity. If the development of arterial hypotension is associated with the described mechanism, the blood volume should be increased. It is recommended to stop using the drug 24 hours before surgery.
Hyperkalemia
During therapy with ACE inhibitors, including perindopril, plasma potassium levels may increase in some patients. Risk factors for the development of hyperkalemia are renal failure, decreased renal function, old age (over 70 years), diabetes mellitus, intercurrent conditions, in particular dehydration, acute cardiac decompensation, metabolic acidosis, simultaneous use of potassium-sparing diuretics (for example, spironolactone, eplerenone, triamterene or amiloride), potassium-containing drugs or supplements, potassium-containing salt substitutes, or concomitant use of other drugs that increase plasma potassium levels (eg, heparin). Hyperkalemia can cause serious, sometimes life-threatening arrhythmias. If it is necessary to use perindopril and one of the above substances simultaneously, caution should be exercised and the potassium level in the blood plasma should be regularly monitored.
Patients with diabetes mellitus
In patients with diabetes mellitus taking oral hypoglycemic agents and/or insulin, increased monitoring of blood glucose concentrations is necessary during the first few months of therapy with ACE inhibitors.
Amlodipine
Liver dysfunction
In patients with impaired liver function, T1/2 of amlodipine is prolonged. When prescribing the drug to such patients, caution should be exercised and the activity of liver enzymes should be regularly monitored.
Patients with heart failure
In patients with CHF (functional class III and IV according to the NYHA classification), treatment is carried out with caution, due to the possibility of developing pulmonary edema.
Pharmacodynamics: how it affects blood pressure
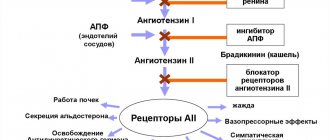
The blood pressure drug Dalneva is a potent antihypertensive drug. The principle of action is the ability of the components of the tablets to suppress ACE enzymes, which provoke the production of angiotensin, which increases blood pressure (BP). Has a vasodilating effect.
Due to the presence of an active metabolite, perindoprilate, Dalneva reduces blood pressure (systolic and diastolic) quickly and effectively.
Expands peripheral arteries, blood vessels in the area of the heart muscle, significantly reducing the level of load on the heart and the myocardium's need for oxygen. Relieves spasmodic phenomena affecting the coronary arteries.
Helps reduce left ventricular hypertrophy without significantly affecting heart rate. Has a mild diuretic effect.
Dalneva presents a combination drug combining the actions of ACE inhibitors and calcium channel blockers
Pharmacokinetics of the drug
The tablets are quickly absorbed, entering the gastrointestinal tract. Pre-meal reduces the bioavailability of the drug. In the liver it is transformed into inactive metabolites.
The drug begins to act, reducing blood pressure, after 4 to 6 hours from the moment of taking the tablets. The hypotensive effect lasts throughout the day, which makes it possible to avoid frequent medication use.
Despite its rapid action, Dalneva at high blood pressure is characterized by a cumulative effect. Therefore, after a month of the therapeutic course, blood pressure levels stabilize, and other signs characteristic of hypertension are eliminated. The drug is excreted from the body along with urine using the renal apparatus.
Drug interactions
When concomitant therapy with other drugs is necessary, it is necessary to take into account the interaction with them of each of the active components of the drug.
With simultaneous use of Dalneva:
- spironolactone, triamterene, amiloride and other potassium-sparing diuretics, potassium supplements, potassium-containing table salt substitutes can cause a significant increase in plasma potassium levels;
- Lithium preparations contribute to an increase in the concentration of lithium in the blood serum and the development of toxic effects;
- Estramustine increases the risk of developing angioedema;
- non-steroidal and non-selective anti-inflammatory drugs, high doses (more than 3 g per day) of acetylsalicylic acid can lead to a decrease in the hypotensive, diuretic, natriuretic effect of perindopril, a deterioration in renal function up to the development of acute renal failure, an increase in the potassium content in the blood serum;
- oral hypoglycemic agents, insulin enhance their effect;
- thiazide and loop diuretics can significantly reduce blood pressure;
- sympathomimetics can reduce the hypotensive effect of the drug;
- gold preparations for injection sometimes cause nausea, vomiting, flushing of the face, and a decrease in blood pressure;
- glucocorticosteroids (GCS) for systemic use, cytostatic and immunosuppressive agents, allopurinol, procainamide increase the risk of leukopenia;
- agents for general anesthesia, tricyclic antidepressants, antipsychotics enhance the hypotensive effect of the drug, increasing the risk of developing orthostatic hypotension;
- dantrolene for intravenous administration increases the risk of hyperkalemia;
- rifampicin, St. John's wort, anticonvulsants (carbamazepine, phenobarbital, fosphenytoin, primidone, phenytoin) can cause increased metabolism of amlodipine and a decrease in its plasma concentration;
- protease inhibitors, azole antifungals (itraconazole, ketoconazole), macrolides (including erythromycin, clarithromycin, diltiazem, verapamil) may increase the plasma concentration of amlodipine and increase the risk of adverse effects;
- beta-blockers (bisoprolol, metoprolol), carvedilol increase the risk of arterial hypotension and enhance the negative inotropic effect of the drug;
- baclofen, vasodilators can potentiate the hypotensive effect of the drug;
- mineralocorticosteroids and corticosteroids, tetracosactide reduce the hypotensive effect of the drug;
- prazosin, alfuzosin, tamsulosin, doxazosin, terazosin (alpha-blockers) enhance the hypotensive effect and increase the risk of orthostatic hypotension;
- amifostine may increase the hypotensive effect of amlodipine;
- cimetidine, sildenafil, grapefruit juice (240 ml) do not affect the pharmacokinetics of amlodipine;
- cyclosporine, atorvastatin, digoxin, warfarin do not change their pharmacokinetic parameters.
Indications for taking tablets
Doctors recommend that patients take Dalneva for high blood pressure in the following clinical cases:
- Arterial hypertension;
- Cardiac ischemia;
- Stable angina;
- Hypertonic disease.
The drug can be used as an independent medicine, however, in case of cardiovascular disorders, the tablets are used as one of the constituent elements of a complex therapeutic course!
The medicine is prescribed for angina pectoris and hypertension
Contraindications
Dalneva is contraindicated in patients in the following cases:
- Renal failure;
- Hypotonic disease (low blood pressure);
- Individual intolerance and hypersensitivity to the ingredients contained in the tablets;
- The patient is a minor;
- Pregnancy period;
- Breastfeeding.
With increased caution, tablets are prescribed to patients with diagnosed atherosclerosis, diabetes mellitus, chronic heart failure, cardiomyopathy, aortic stenosis, lupus erythematosus, mitral stenosis, and liver dysfunction.
Instructions for use and dosage
Instructions for use Dalnevs recommend taking the tablets once a day, before meals, preferably in the morning.
The optimal dosage of the drug is selected by the doctor individually, depending on the diagnosis, severity of the disease and other features of a particular clinical case. The maximum daily dose of the drug should not exceed 1 tablet (perindopril - 8 mg, amlodipine - 10 mg).
To achieve a stable, maximally pronounced therapeutic effect, it is recommended to drink Dalneva regularly for at least one month.
Release form and composition
Dalneva is available in the form of almost white or white tablets: 5 mg + 4 mg – slightly biconvex round, with a chamfer; 10 mg + 4 mg – biconvex capsule-shaped, on one side there is a dividing line; 5 mg + 8 mg – round biconvex shape, with a chamfer; 10 mg + 8 mg – round, biconvex shape, with a chamfer and a dividing line on one side (10 pcs. in a blister pack, 3 or 9 packs in a cardboard box).
1 tablet contains:
- active ingredients: amlodipine besylate + perindopril erbumine A (in the form of granules) – 6.935 mg + 21 mg, 13.87 mg + 21 mg, 6.935 mg + 42 mg or 13.87 mg + 42 mg, which is equivalent to the content of 5 mg + 4 mg, 10 mg + 4 mg, 5 mg + 8 mg or 10 mg amlodipine + 8 mg perindopril erbumine;
- auxiliary components: microcrystalline cellulose, pregelatinized starch, sodium carboxymethyl starch, sodium bicarbonate, colloidal silicon dioxide, magnesium stearate.
Adverse reactions
Treatment with Dalneva tablets can cause the following side effects:
- Nausea and vomiting;
- A sharp decrease in blood pressure;
- Dyspnea;
- Painful sensations localized in the abdomen;
- Dyspeptic disorders;
- Fainting conditions;
- Increased heart rate (tachycardia);
- Swelling;
- Erectile dysfunction in male patients;
- Frequent urge to urinate;
- Diarrhea;
- Noise and ringing in the ears;
- Tremor;
- Dizziness;
- Migraine attacks;
- Cough syndrome;
- Constipation;
- Skin itching;
- Deterioration of visual function;
- Myalgia;
- Chronic fatigue, lethargy, asthenia;
- Allergic rashes on the skin;
- Myalgia;
- Muscle spasms.
Any side effects that occur during the therapeutic course must be reported to your doctor! Before consulting with a specialist, you should not stop taking the drug or replace it with one of its analogues.
Taking the drug may cause migraine attacks
Side effects
- from the circulatory and lymphatic systems: very rarely - agranulocytosis, leukopenia or neutropenia, thrombocytopenia, pancytopenia, with congenital deficiency of glucose-6-phosphate dehydrogenase - hemolytic anemia, decreased hematocrit and hemoglobin concentrations;
- from the cardiovascular system: often – a pronounced decrease in blood pressure, a rush of blood to the skin of the face, a feeling of palpitations; infrequently – fainting; rarely – chest pain; very rarely - angina pectoris, myocardial infarction, arrhythmias (including bradycardia, atrial fibrillation, ventricular tachycardia), stroke, vasculitis;
- from the immune system: infrequently – urticaria;
- from the nervous system: often – headache, dizziness, drowsiness, vertigo, paresthesia; uncommon – sleep disturbance, insomnia, tremor, mood lability, hypoesthesia; very rarely - confusion, peripheral neuropathy;
- metabolic disorders: infrequently - increase or decrease in body weight; very rarely - hyperglycemia; frequency unknown - hypoglycemia;
- from the senses: often – tinnitus, visual impairment;
- from the digestive system: often – dyspepsia, nausea, vomiting, abdominal pain, constipation, diarrhea; infrequently - dryness of the oral mucosa, disturbance of the sense of taste, disturbance of the rhythm of bowel movements; very rarely - gastritis, gingival hyperplasia, pancreatitis, cholestatic jaundice, hepatitis, cholestatic or cytolytic hepatitis;
- from the respiratory system: often – cough, shortness of breath; uncommon – bronchospasm, rhinitis; very rarely - eosinophilic pneumonia;
- from the musculoskeletal system: often – muscle spasms; uncommon – back pain, arthralgia, myalgia;
- on the part of the skin: often – itching, rash; uncommon – angioedema of the vocal folds and/or larynx, tongue, lips, mucous membranes, face or extremities, photosensitivity, hemorrhagic rash, excessive sweating, alopecia; very rarely - erythema multiforme, angioedema, Stevens-Johnson syndrome;
- from the reproductive system: infrequently – gynecomastia, impotence;
- from the urinary system: infrequently - nocturia, urinary dysfunction, renal failure, frequent urination; very rarely - acute renal failure;
- laboratory parameters: rarely - increased bilirubin levels; very rarely - increased activity of alanine aminotransferase (ALT) and aspartate aminotransferase (AST), often in combination with cholestasis; frequency unknown - increased levels of creatinine and urea in the blood serum;
- other: often – asthenia, peripheral edema, increased fatigue; infrequently – malaise, chest pain.
How to replace Dalneva
People suffering from hypertension can find and purchase in pharmacy chains the following analogues of Dalneva:
- Ko-Dalneva;
- Tarka;
- Liten;
- Pristan;
- Amzzar;
- Tenliza;
- Amlodipine;
- Duplecore;
- Rasilam;
- Equacard;
- Amlong;
- Iruzid;
- Vamsolet;
- Kalchek;
- Lisinopril;
- Prestance.
Dalneva tablets are an effective and affordable drug intended for the treatment of hypertension and cardiac ischemia. The drug not only stabilizes blood pressure, but also improves the patient’s condition, improves his overall quality of life, and most importantly, prevents the development of deadly complications!