Brief information about the drug
The blood pressure medicine Capozide belongs to the pharmacological group of drugs - ACE inhibitors. Produced by the American company Bristol-Myers Squibb Company.
Release form
The medication Capozide for blood pressure is presented on the pharmaceutical market in tablet form, which is ideal for use at home. The tablets have a notch indicating the dosage of the main active ingredient. The drug is sold in packages containing 14 tablets.
Compound
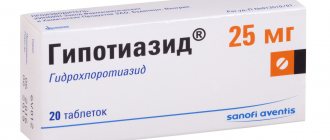
The main active components of the drug are hydrochlorothiazide (25 mg) and captopril (50 mg).
The tablets contain auxiliary elements such as microcrystalline cellulose, magnesium stearate, starch, lactose, and stearic acid.
Packaging of Capozide tablets
pharmachologic effect
The drug Capozide has pronounced hypotensive properties and also acts as a diuretic.
Storage conditions and periods
It is recommended to store the tablets in a place protected from moisture at a temperature not exceeding +25°C. The maximum shelf life of the drug is 3 years.
Conditions for dispensing from a pharmacy
You can purchase the drug in pharmacy chains by presenting the appropriate medical prescription to the pharmacist.
What is the price
The exact price of the drug is determined by factors such as the number of tablets in the pack and the policy of a particular pharmacy chain. The average cost of Capozide tablets is about 280-300 rubles for 30 pieces.
Which is better: Capozide or Capoten?
We often hear the question of which drug to choose - Capozide or Capoten, which is better? It makes sense to prescribe Capozide if Capoten does not have the required effect. Capozide, according to the instructions for use, is a substance that, in addition to the antihypertensive base, contains a diuretic.
It is known that diuretic drugs have a pronounced effect of lowering blood pressure, and in combination with blood pressure-lowering drugs, they enhance the effect of the latter. Therefore, Capoten acts more gently, while Kaposide will reduce blood pressure more sharply and will not allow it to rise for a long time. Capozide makes sense to use at higher blood pressure, which is difficult to normalize with “light” drugs.
Pharmacodynamics - how it affects blood pressure
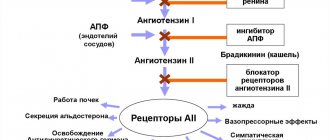
Capozide blood pressure tablets are a combination drug with antihypertensive and diuretic properties. Suppresses the production of angiotensin. Promotes the expansion of large arteries by activating coronary and renal blood flow. It is thanks to these pharmacological properties that Capozide reduces blood pressure.
The drug reduces the load on the myocardium and stabilizes the heart rate. Suppresses the processes of aggregation of platelet cell structures.
With regular use, Capozide effectively reduces the manifestations of myocardial hypertrophy.
It has a moderate diuretic effect, removing excess fluid from the body, eliminating swelling and congestion.
Capozide combines the action of an ACE inhibitor with a diuretic
Composition of the tablet
The tablet consists of captopril and hydrochlorothiazide. It has a more dilating effect on arteries than on veins. It has a strengthening effect on blood circulation, both coronary and renal blood flow. If use is long-term:
- nutrition of the myocardium and arterial walls is normalized;
- blood supply to the myocardium improves after ischemia;
- platelet aggregation decreases.
Hydrochlorothiazide is a medium-strength diuretic that removes sodium from the body. Normalizes blood pressure.
Pharmacokinetics
The drug for high blood pressure Capozide has a rapid antihypertensive effect, while having a cumulative effect. It is well absorbed once it enters the gastrointestinal tract and has high bioavailability rates (about 70%). The maximum concentration of active substances in the blood is reached an hour after taking the tablets.
It is excreted from the body along with urine, using the renal apparatus (about 50%), and partially in the form of metabolites.
What are Capozide tablets prescribed for?
Medical experts recommend drinking Capozide for high blood pressure in patients who have the following clinical indications for the use of the drug:
- Arterial hypertension;
- Tendency to increase blood pressure;
- Diseases of a cardiac nature that occur against the background of hypertension.
Tablets are prescribed to patients diagnosed with hypertension who require a combined therapeutic course.
Contraindications
According to the instructions for use, Capozide is contraindicated for:
- cardiogenic shock;
- excessive sensitivity to captopril, antihypertensive diuretics, sulfonamides;
- narrowing of the aorta and mitral valve;
- cardiomyopathy;
- narrowing of the arteries of the kidneys on both sides;
- with cardiac insufficiency;
- hypotension;
- increased heart rate;
- severe liver pathologies;
- severe kidney disorders, anuria;
- primary hyperaldosteronism;
- pregnancy and breastfeeding.
Capozide is prescribed carefully according to the instructions for use and the patient’s condition is carefully monitored for:
- moderate kidney disorders;
- protein in urine;
- reduction of K ions;
- decreased sodium;
- hypovolemia;
- increased calcium;
- gout;
- immune pathologies;
- old age;
- when treated with drugs that reduce the body's defenses (immunosuppressants, cytostatics, glucocorticosteroids), as well as lithium, procainamide and allopurinol.
Contraindications of the drug
It is strictly forbidden to take Capozide with high blood pressure in patients with the following clinical contraindications:
- Pathologies of the renal apparatus that occur in severe form;
- Arterial hypotension (low blood pressure);
- Obstructive cardiomyopathy of a hypertrophic nature;
- Individual intolerance and excessive sensitivity to the components contained in the drug;
- Hepatic coma;
- Mitral stenosis;
- The presence in the patient's medical history of angioedema caused by taking ACE inhibitors;
- Shock state of cardiogenic nature;
- Rapid heartbeat (tachycardia);
- Previous surgery for a kidney transplant;
- Hyperaldosteronism;
- Chronic heart failure;
- Bilateral renal artery stenosis;
- Aortic stenosis.
Tablets are prohibited from being used to treat women expecting the birth of a baby, mothers during breastfeeding, and children and adolescents who have not reached the age of majority.
Chronic heart failure is a contraindication for taking the drug
With particular caution, Capozide for high blood pressure is prescribed to persons over the age of 65 years, to patients suffering from:
- hypercalcemia;
- periarteritis;
- hypokalemia;
- scleroderma;
- gout;
- proteinuria;
- hyponatremia;
- diseases of the renal apparatus;
- systemic pathologies of connective tissue structures;
- diabetes mellitus;
- autoimmune diseases,
Treatment of this category of patients requires individual selection of a safe dose and strict supervision by specialists.
It is not recommended to use Capozide during therapy with cytostatics, immunosuppressants, Procainamide, lithium preparations, Allopurinol.
Capozide
Use during pregnancy and breastfeeding
The use of Kaposide® is contraindicated during pregnancy.
Epidemiological data demonstrating a risk of teratogenicity after exposure to ACE inhibitors in the first trimester of pregnancy have not been convincing, but some increased risk cannot be excluded. If the use of an ACE inhibitor is considered necessary, patients planning pregnancy should be switched to alternative antihypertensive therapy that has an established safety profile for use in pregnancy. It is known that long-term exposure of the fetus to ACE inhibitors in the second and third trimesters of pregnancy can lead to disruption of its development (decreased renal function, oligohydramnios, delayed ossification of the skull bones) and the development of complications in the newborn (such as renal failure, arterial hypotension, hyperkalemia) . If the patient received the drug Capozide® in the second and third trimesters of pregnancy, it is recommended to conduct an ultrasound examination to assess the condition of the skull bones and fetal kidney function.
The use of hydrochlorothiazide during pregnancy is not recommended as it may impair placental perfusion and cause fetal/neonatal jaundice, thrombocytopenia, fluid and electrolyte imbalance and possibly other adverse reactions observed in adults.
The use of ACE inhibitors during pregnancy can cause developmental disorders and fetal death. If pregnancy is established, the use of the drug Capozide should be discontinued as soon as possible.
Captopril and hydrochlorothiazide are found in breast milk after oral administration by a nursing woman. Due to the risk of serious adverse reactions in the child caused by both active substances, breastfeeding should be stopped or therapy with Capozide® should be discontinued in the mother during the period of breastfeeding.
Use for liver dysfunction
Contraindicated in severe liver failure (precomatose state, hepatic coma).
The drug should be prescribed with caution in case of liver dysfunction or progressive liver diseases.
Use for renal impairment
Contraindicated in severe renal failure (serum creatinine >1.8 mg/dL or creatinine clearance <20-30 ml/min, anuria).
The drug should be prescribed with caution in case of moderate renal failure (creatinine clearance 30-60 ml/min).
Use in children
The drug is contraindicated in children and adolescents under 18 years of age.
special instructions
At the beginning of treatment, an excessive decrease in blood pressure may be observed, especially in patients with chronic heart failure, severe arterial hypertension (including renal origin) and/or renal failure. Before starting treatment, it is necessary to compensate for the deficiency of sodium ions and normalize the blood volume (reduce the dose of previously prescribed diuretics or, in some cases, completely cancel them), and also determine indicators of kidney function.
Regular monitoring of the concentration of potassium and calcium in the blood serum is necessary (especially in patients receiving treatment with cardiac glycosides, glucocorticoids, often using laxatives, as well as in elderly patients), glucose, uric acid, lipids (cholesterol and triglycerides), urea and creatinine, activity liver enzymes.
Regular monitoring of blood pressure and laboratory parameters is especially necessary in the following cases: in patients with renal failure; patients with severe arterial hypertension (including renal origin); in elderly patients (over 65 years old); in patients with water-electrolyte imbalance and decompensated chronic renal failure; as well as those receiving simultaneously allopurinol, lithium salts, procainamide and drugs that reduce immunity.
When using ACE inhibitors, a characteristic non-productive cough is observed, which ceases after discontinuation of ACE inhibitor therapy.
Some patients with kidney disease, especially those with severe renal artery stenosis, experience increases in serum urea nitrogen and creatinine concentrations after lowering blood pressure. This phenomenon is usually reversible; a decrease in the concentration of urea nitrogen and creatinine in the blood serum is observed after discontinuation of the drug.
In some cases, during the use of ACE inhibitors, an increase in the concentration of potassium in the blood serum is observed. The risk of developing hyperkalemia when using ACE inhibitors is increased in patients with renal failure and diabetes mellitus, as well as those taking potassium-sparing diuretics, potassium supplements or other drugs that cause an increase in the concentration of potassium in the blood (for example, heparin). The simultaneous use of potassium-sparing diuretics and potassium supplements should be avoided. In addition, when using ACE inhibitors simultaneously with thiazide diuretics, the risk of hypokalemia cannot be excluded, therefore, in such cases, regular monitoring of potassium concentrations in the blood during therapy should be carried out.
When performing hemodialysis in patients receiving ACE inhibitors, the use of high-permeability dialysis membranes (for example, AN69) should be avoided, as in such cases the risk of developing anaphylactoid reactions increases. Anaphylactoid reactions have also been observed in patients undergoing LDL apheresis with dextran sulfate. The use of either a different class of antihypertensive drugs or a different type of dialysis membrane should be considered.
If angioedema develops, the drug is discontinued and careful medical observation is carried out until the symptoms disappear completely. Angioedema of the larynx can be fatal. If the swelling is localized on the face, special treatment is usually not required (antihistamines can be prescribed to reduce the severity of symptoms); if the swelling spreads to the tongue, pharynx or larynx and there is a threat of developing airway obstruction, epinephrine (adrenaline) should be immediately administered subcutaneously (0.3-0.5 ml in a dilution of 1:1000). In rare cases, patients after taking ACE inhibitors experienced angioedema of the intestine, which was accompanied by abdominal pain (with or without nausea and vomiting), sometimes with normal values of C1-esterase activity and without previous facial edema. Intestinal edema should be included in the differential diagnosis in patients complaining of abdominal pain while taking ACE inhibitors. In two patients undergoing desensitization with hymenoptera venom, life-threatening anaphylactoid reactions were noted while taking captopril. When the ACE inhibitor was temporarily discontinued in the same patients, anaphylactoid reactions were avoided. Caution should be exercised when performing desensitization in patients taking ACE inhibitors.
In patients with diabetes mellitus receiving oral hypoglycemic agents or insulin, blood glucose concentrations should be carefully monitored, especially during the first month of ACE inhibitor therapy.
ACE inhibitors are less effective in blacks than in Caucasians, which may be due to the higher prevalence of low renin activity in blacks.
During major surgery or during anesthetic therapy, patients taking ACE inhibitors may experience an excessive decrease in blood pressure. In these cases, measures are taken to increase the BCC.
In rare cases, when taking ACE inhibitors, a syndrome is observed that begins with the appearance of cholestatic jaundice, progressing to fulminant hepatonecrosis, sometimes with a fatal outcome. The mechanism of development of this syndrome is unknown. If a patient receiving ACE inhibitor therapy develops jaundice or a marked increase in liver enzymes, treatment with ACE inhibitors should be discontinued and the patient should be monitored.
Neutropenia/agranulocytosis, thrombocytopenia and anemia have been reported in patients taking ACE inhibitors. Neutropenia is rare in patients with normal renal function and no other abnormalities.
The drug Capozide® should be used very carefully in patients with autoimmune connective tissue diseases, in patients taking immunosuppressants, allopurinol and procainamide, especially in the presence of pre-existing renal dysfunction. Due to the fact that the majority of fatal cases of neutropenia during the use of ACE inhibitors developed in such patients, their leukocyte count should be monitored before starting treatment, in the first 3 months - every 2 weeks, then every 2 months.
In all patients, the number of leukocytes in the blood should be monitored monthly in the first 3 months after starting therapy, then every 2 months. If the number of leukocytes is below 4000/μl, a repeat general blood test is indicated; if below 1000/μl, the drug is stopped while monitoring the patient continues. Typically, recovery in neutrophil numbers occurs within 2 weeks after discontinuation of captopril. In 13% of cases of neutropenia, death was noted. In almost all cases, fatal neutropenia was observed in patients with connective tissue diseases, renal or heart failure, while taking immunosuppressive drugs, or a combination of these factors.
When using ACE inhibitors, proteinuria may occur, mainly in patients with impaired renal function, as well as when using drugs in high doses. In most cases, proteinuria disappeared or decreased in severity after taking captopril within 6 months, regardless of whether the drug was stopped or not. Renal function tests (blood urea nitrogen and creatinine concentrations) in patients with proteinuria were almost always within normal limits. In patients with kidney disease, the concentration of protein in the urine should be determined before starting treatment and periodically throughout the course of therapy.
Sulfonamide derivatives (including hydrochlorothiazide) can cause transient myopia and acute angle-closure glaucoma, risk factors include a history of allergy to sulfonylureas or penicillin. Symptoms (sharp decrease in visual acuity, pain in the eyeball) are usually observed within a few hours to several weeks after the start of treatment. If symptoms appear, you should immediately stop taking the drug; If necessary, medications to correct intraocular pressure should be prescribed.
In all patients taking thiazide diuretics, clinical signs of fluid and electrolyte imbalance (hyponatremia, hypochloride alkalosis, hypokalemia) should be identified. It is especially important to determine the content of electrolytes in the blood serum and urine during excessive prolonged vomiting or during the administration of infusion solutions. Signs of water and electrolyte imbalance may include dry mouth, thirst, weakness, lethargy, confusion, anxiety, muscle pain or cramps, muscle weakness, excessive decrease in blood pressure, oliguria, tachycardia, nausea, vomiting.
Hypokalemia can provoke or enhance the cardiotoxic effect of cardiac glycosides.
The deficiency of chlorine ions is usually mild and does not require correction.
Patients with edema in hot weather may experience hyponatremia caused by an increase in blood volume. Liquid intake should be limited. In cases of life-threatening hyponatremia, sodium chloride solution is prescribed.
Hyperuricemia or gout attacks may occur during therapy with thiazide diuretics; Latent diabetes mellitus may also manifest itself.
Thiazide derivatives can cause a decrease in the concentration of bound iodine in the blood serum without signs of thyroid dysfunction.
While taking thiazide diuretics, the degree of calcium excretion decreases; There have been cases of pathological changes in the parathyroid glands, accompanied by hypercalcemia and hypophosphatemia. Before monitoring parathyroid function, the thiazide diuretic should be discontinued.
While taking thiazide diuretics, there was an increase in the degree of magnesium excretion, which can lead to hypomagnesemia.
The drug Capozide® may cause a false-positive reaction when testing urine for ketone bodies and distort the results of the test with bentiromide.
If fever appears, swollen lymph nodes and/or signs of laryngitis and/or pharyngitis develop, the white blood cell count must be immediately determined.
The use of thiazide diuretics may cause a positive result during doping control.
Impact on the ability to drive vehicles and operate machinery
During the treatment period, care must be taken when driving vehicles and engaging in other potentially hazardous activities that require increased concentration and speed of psychomotor reactions.
Instructions for use and dosage
Instructions for use of Capozide recommend taking the tablets once a day, an hour before meals.
It is recommended to start treatment with half a tablet, increasing the daily dose after a week of the therapeutic course.
The duration of therapy is determined by the doctor individually depending on blood pressure, the stage of development of hypertension, the body's reactions and other features of a particular clinical case.
The tablets are drunk whole without preliminary crushing or chewing.
Capozide tablets should not be crushed; the tablets should be taken whole
Patient reviews
Users' opinions about the drug are necessary both for patients to know what to expect from this drug, and for manufacturers and distributors to understand what still needs to be worked on. Capozide is no exception to this.
Reviews from patients are varied, but mostly they talk about positive results from taking the drug.
So Alexandra shared that her mother took Capozide tablets for a long time. I liked the result. There were no side effects while taking the drug. The only undesirable manifestation was at the beginning of treatment: the pressure dropped sharply. But this was the only time, subsequent appointments went well.
Irina noticed that the pills helped her a lot. They brought her blood pressure to a stable state, and she also noted a significant improvement in her health. From time to time there was slight nausea, which quickly passed.
But opinions vary, there are also negative ones. For example, one doctor said that Capozide is outdated and does not have a sufficiently long-lasting effect on the patient’s body.
The patient, who has been taking the drug for four months, also agrees with him, but has not received the expected normalization of blood pressure, which is indicated in the instructions for use. In addition, she developed a dry cough, dizziness, and nausea.
Some patients taking Capozide give reviews with complaints about the side effects of the drug indicated in the instructions for use. Some patients experienced edema, tachycardia, blood pressure dropped too sharply (especially in the morning), and a dry cough. One fifty-five-year-old man complains of skin rashes that appear and disappear.
We can conclude that Capozide, like many other antihypertensive drugs, should be tried to be included in the treatment regimen. If it works effectively, the dosage should be adjusted based on the patient's condition.
Manifestations of adverse reactions
When taking Capozide for high blood pressure, you need to be aware of the following side effects that the drug can cause:
- Dizziness;
- Excessive decrease in blood pressure;
- Hyperkalemia;
- Erectile dysfunction in male patients;
- Convulsive syndrome;
- Headache;
- General weakness, malaise;
- Decreased performance, chronic fatigue syndrome;
- Stool disorders (constipation, diarrhea);
- Painful sensations localized in the abdomen;
- Nausea and vomiting;
- Dermatitis;
- Hair loss;
- Muscular and joint pain;
- Permanent lack of appetite;
- Sleep disorders, insomnia;
- Bronchial spasms;
- Increased heart rate;
- Edema of an allergic nature;
- Hives;
- Tremor;
- Noise in ears;
- Visual impairment (myopia);
- Paresthesia;
- Ataxia;
- Cough syndrome;
- Hyperglycemia;
- Depressive states;
- Anemia;
- Rhinitis, sinusitis;
- Feeling of dry mouth and constant thirst.
In rare cases, the development of vasculitis, hepatitis, cholecystitis, nephritis, and renal dysfunction is possible.
Any adverse reactions must be reported to the attending physician in order to adjust the daily dosage of tablets or select a more suitable analogue.
Dermatitis may be one of the adverse reactions from the use of the drug
Side effects
The use of Capozide may cause side effects from certain body systems, namely:
- Bronchospasm, rhinitis, respiratory failure, laryngitis, dry cough, sinusitis (respiratory system);
- Disturbances in water-electrolyte balance, decreased formation of tear fluid (water-electrolyte metabolism);
- Dizziness, fever, flushing of the face, headache, marked decrease in blood pressure, swelling of the legs, feeling of weakness, fainting, tachycardia, palpitations, stroke, myocardial infarction, Raynaud's syndrome (cardiovascular system);
- Angioedema of the tongue, mucous membranes, limbs, lips, face, larynx and pharynx, skin rash, urticaria (allergic reactions);
- Exfoliative dermatitis, exudative erythema multiforme and toxic epidermal necrolysis, accompanied by the development of vasculitis, increased body temperature, pain in muscles and joints (dermatological reactions);
- Nausea, vomiting, reversible and usually self-limiting disturbance of the sense of taste, stomatitis, constipation or diarrhea, decreased appetite, discomfort in the stomach, acute cholecystitis, abdominal pain, hemorrhagic pancreatitis, increased activity of liver enzymes, hepatitis, cholestatic jaundice, asthenia, hyperbilirubinemia, intestinal angioedema, gingival hyperplasia (digestive system);
- Hyperuricemia, hypermagnesemia, hyperglycemia, hyperlipidemia (metabolism);
- Oliguria, proteinuria, polyuria, glycosuria, increased frequency of urination, impotence, hyponatremia, increased serum concentrations of urea, creatinine and potassium ions, nephritis, impaired renal function (urinary system);
- Ataxia, feeling of fatigue, sleep disturbances, headache, depression, decreased potency, convulsions, paresthesia, depression, impaired sense of balance, impaired visual acuity, dizziness, tinnitus, tremor, taste disturbances, progression of myopia (central and peripheral nervous system) ;
- Decreased hematocrit, anemia, leukopenia, thrombocytopenia, neutropenia, eosinophilia, increased titer of antinuclear antibodies (hematopoietic system).
Capozide overdose
The use of Capozide tablets in excessively high dosages leads to a more pronounced manifestation of the side effects characteristic of this drug. The following pathological conditions may develop:
- Hypotonic crisis, provoked by a sharp and excessive decrease in blood pressure;
- Slow heartbeat (bradycardia);
- Violation of water and electrolyte balance;
- Stupor;
- State of shock:
- Cardiac dysfunction;
- Depression of respiratory function.
An overdose of Capozide can cause the patient to fall into a coma and even die, so it is important to provide the victim with competent assistance in a timely manner. To do this, the patient is given a gastric lavage and given a portion of sorbents. In especially severe cases, hospitalization, intravenous drips, and hemodialysis may be required.
Further therapy is symptomatic and is prescribed by a doctor on an individual basis.
special instructions
At the beginning of use according to the instructions for use, a rapid drop in blood pressure is possible, especially in people with kidney failure or suffering from cardiac failure or severe hypertension.
Before starting therapy with Capozide tablets, it is necessary to normalize Na+ and BCC (reduce the doses of previously prescribed diuretics or abandon them), and also undergo a kidney examination.
It is necessary to constantly monitor K and Ca in the blood (especially if patients are taking digitalis-based drugs, adrenal hormones, constipation drugs, as well as in old age), sugar, uric acid, fats, kidney tests, ALT and AST.
During use, blood pressure and laboratory tests are monitored in the following cases:
- kidney failure;
- severe hypertension;
- in old age (more than sixty-five years);
- if water-electrolyte metabolism is disturbed;
- with severe cardiac failure;
- taking simultaneously allopurinol, lithium-containing drugs, procainamide and drugs that weaken the body's immune system.
Capozide, a high blood pressure medication, requires you to avoid activities that require you to be alert, focused, and quick to react.
Drug compatibility
The combination of Capozide with thiazide diuretics, beta-blockers, antidepressants, and sleeping pills enhances the hypotensive effect and threatens the development of a hypotensive crisis.
The combination of tablets with Methenamine, Cholestyramine, COX inhibitors, Indomethacin, on the contrary, significantly reduces the antihypertensive effect of Capozide.
Use together with Cimetidine helps to increase the concentration of the active components of Capozide in the blood, which must be taken into account when selecting the optimal dosage of the drug.
Simultaneous use of the drug with diuretics and diuretics, cardiac glucosides, increases the manifestation of undesirable reactions characteristic of these medications.
Capozide significantly enhances the effect of anesthetic drugs, which is important to consider when planning surgery.
You should check with your doctor about the compatibility of Capozide with other drugs.
Analogs are cheaper
Nowadays, antihypertensive drugs are quite in demand, among them is Capozide. Many patients want to find cheaper analogues. Caposide has no exact analogues: it is the only drug containing captopril and hydrochlorothiazide. A cheaper replacement for the product can be called: Co-Diroton, Co-Renitek, Lisinoton N.
It is not recommended to independently replace one remedy with another, even if it is similar in action. Such prescriptions should only be made by a doctor.