Compound
One Femoston 1/5 tablet contains estradiol and dydrogesterone in concentrations of 1 and 5 mg, respectively.
The auxiliary components used are: lactose in the form of monohydrate, methylhydroxypropylcellulose, anhydrous colloidal silicon dioxide, corn starch, macrogol 400, magnesium stearate, iron dyes (yellow oxide E172 and red E172), titanium dioxide (E171), Opadry orange. Femoston Conti 1/5 tablets have a similar composition.
Femoston 1/10 white tablets use estradiol . Substance concentration - 1 mg/tab. Each gray Femoston tablet contains estradiol and dydrogesterone estradiol per 10 mg dydrogesterone ).
Femoston 2/10 pink tablets contain estradiol at a concentration of 2 mg/tablet. Light yellow tablets contain estradiol and dydrogesterone estradiol per 10 mg dydrogesterone ). Auxiliary components: lactose in the form of monohydrate, hypromellose, magnesium stearate, corn starch, colloidal silicon dioxide, Opadry (white, gray, pink and yellow, respectively).
Release form
The dosage form of the drug is film-coated, round, biconvex tablets with a diameter of 0.7 cm. The tablets differ in color depending on the concentration of the active substance/substances; each of them is marked “379” on one side.
Femoston 1/5 tablets have the letter “S” engraved on the other side. Tablets are available in calendar packs of 28 pieces.
Tablets with a higher concentration of active substances are packaged in calendar packs as follows:
- 14 white tablets 1 mg + 14 gray tablets 1 mg + 10 mg (Femoston 1/10);
- 14 pink tablets 2 mg + 14 light yellow tablets 2 mg + 10 mg (Femoston 2/10).
Drug interactions
When used simultaneously with Femoston 1/10:
- carbamazepine, phenobarbital, phenytoin (anticonvulsants), rifabutin, nevirapine, rifampicin, efavirenz (antimicrobials), St. John's wort, ritonavir, nelfinavir: increase the metabolism of the active components of the drug, which may result in a decrease in its therapeutic effect and a change in the intensity of vaginal bleeding ;
- tacrolimus, cyclosporine, theophylline, fentanyl: can significantly increase their plasma concentration levels.
Pharmacodynamics and pharmacokinetics
Femoston is a combined hormonal drug used to eliminate the symptoms of estrogen deficiency and treat DUB - dysfunctional uterine bleeding .
The estradiol contained in the drug is identical to endogenous estradiol . The drug is used to replenish estrogen after the onset of menopause and effectively treats vegetative and psychoemotional disorders associated with menopause , accompanied by:
- hyperhidrosis;
- tides;
- involution of the mucous membranes and skin, and especially the mucous membranes of the urogenital tract (in particular, the vaginal mucosa, due to which a woman begins to experience discomfort during sexual intercourse);
- increased nervous excitability;
- headaches and dizziness;
- sleep disorders;
- loss of bone mass or osteoporosis (especially if certain risk factors are noted - long-term treatment with glucocorticosteroids in the recent past, early onset of menopause , asthenic type of build, smoking, etc.).
Estradiol also helps reduce the concentration of total cholesterol and low-density lipoproteins, while simultaneously increasing the concentration of high-density lipoproteins.
The action of the gestagenic component of the drug - dydrogesterone - is aimed at stimulating the onset of the secretory phase of the endometrial cycle, and also reduces the risk of carcinogenesis and endometrial hyperplasia associated with the influence of estrogen .
Dydrogesterone does not have androgenic estrogenic , glucocorticosteroid or anabolic effects . To ensure the maximum preventive effect of hormone replacement therapy (HRT), treatment is recommended to begin as early as possible after the onset of menopause .
After taking p/os, estradiol is easily absorbed. Biotransformation of the substance occurs in the liver ; the metabolic are estrone and estrone in the form of sulfate . Estradiol and estrone glucuronides are eliminated from the body primarily in the urine.
Dydrogesterone is also rapidly absorbed from the digestive tract after oral administration. The substance is completely biotransformed, the main product of metabolism is 20-dihydrodydrogesterone. Metabolites excreted mainly through urine.
The half-life of dydrogesterone is from 5 to 7 hours, its main metabolite is from 14 to 17 hours, the substances are completely eliminated after 72 hours.
Pharmacological properties
Pharmacodynamics
Femoston 1/10 is a combination drug intended for hormone replacement therapy (HRT) to prevent bone loss in the postmenopausal period and after oophorectomy. The identity of estradiol hemihydrate to endogenous human estradiol, which is the most active estrogen, makes it possible to compensate for the deficiency of estrogen in the body of a woman at menopausal age and reduces the symptoms of menopause during the first weeks of using the drug. The therapeutic effectiveness of dydrogesterone (progestogen) is similar in activity to the action of progesterone for parenteral administration. Dydrogesterone during HRT ensures complete secretory transformation of the endometrium. Its presence in the drug reduces the risk of developing endometrial hyperplasia, which is increased by the action of estrogens.
Pharmacokinetics
After taking Femoston 1/10 orally, micronized estradiol and dydrogesterone are rapidly and completely absorbed from the gastrointestinal tract. The bioavailability of dydrogesterone is 28%, the maximum concentration in the blood plasma occurs after 0.5–2.5 hours.
Binding to blood plasma proteins: estradiol - approximately 98-99% (with albumin - 30-52%, with globulin - up to 69%), dydrogesterone - more than 90% of the dose taken.
In the liver, estradiol is metabolized to estrone and estrone sulfate, which have estrogenic activity. Estrone sulfate also has the ability of enterohepatic recirculation.
Dydrogesterone is completely metabolized, its main metabolite is 20-a-dihydrodydrogesterone (DHD), the maximum concentration in the blood plasma is reached approximately 1.5 hours after taking Femoston 1/10. The plasma concentration of DHD significantly exceeds the initial level of dydrogesterone concentration.
The lack of estrogenic and androgenic activity is determined by the characteristic feature of all dydrogesterone metabolites to retain the 4,6-dien-3-one configuration of the original substance and the absence of 17alpha-hydroxylation.
The half-life for dydrogesterone is 5–7 hours (DGD is 14–17 hours), estrone and estradiol is 10–16 hours.
Estrogens pass into breast milk.
Estrone and estradiol in a state conjugated with glucuronic acid are excreted primarily through the kidneys.
Approximately 63% of the dose of dydrogesterone is excreted by the kidneys; its complete elimination occurs only after 72 hours. Its total plasma clearance is 6.4 l/min. DHD is detected in urine primarily as a glucuronic acid conjugate.
With daily intake of Femoston 1/10, the equilibrium concentration of estradiol occurs after 5 days, dydrogesterone - after 3 days.
The pharmacokinetic properties of dydrogesterone and DHD do not change with repeated administration.
Indications for use
The use of Femoston is indicated for hormone replacement therapy to eliminate phenomena caused by estrogen deficiency in postmenopausal .
The medicine is prescribed no earlier than six months after the last menstrual bleeding.
Prophylactic use of the drug is advisable to prevent the development of osteoporosis after the onset of menopause . The drug is prescribed to women who have an increased risk of fractures and who are contraindicated in the use of other medications intended to prevent bone loss.
pharmachologic effect
A combined two-phase drug for hormone replacement therapy, containing micronized 17β-estradiol as an estrogenic component and dydrogesterone as a gestagenic component. Both components are analogues of female sex hormones (estradiol and progesterone).
Estradiol replenishes the deficiency of estrogen in the female body after menopause and provides effective relief of psycho-emotional and vegetative menopausal symptoms, such as hot flashes, increased sweating, sleep disturbances, increased nervous excitability, dizziness, headache, involution of the skin and mucous membranes, especially the genitourinary system (dryness) and irritation of the vaginal mucosa, pain during sexual intercourse).
Hormone replacement therapy (HRT) with Femoston ® prevents bone loss in the postmenopausal period caused by estrogen deficiency.
Taking Femoston ® leads to a change in the lipid profile towards a decrease in the level of total cholesterol and LDL and an increase in HDL.
Dydrogesterone is a gestagen, effective when taken orally, which completely ensures the onset of the secretion phase in the endometrium, thereby reducing the risk of developing endometrial hyperplasia and/or carcinogenesis (increased with the use of estrogens). Dydrogesterone does not have estrogenic, androgenic, anabolic or glucocorticoid activity.
Contraindications
The drug is not prescribed:
- women who have been diagnosed with malignant estrogen- or progestogen-dependent tumors , as well as if these diseases are suspected;
- patients with diagnosed or suspected breast cancer ;
- with vaginal bleeding of unspecified origin;
- with untreated hyperplasia (pathological growth) of the endometrium ;
- venous thromboembolism detected at the moment or noted in the anamnesis (including DVT and PE);
- if the patient has certain thrombophilic disorders (including thrombophilia associated with deficiency of antithrombin , coagulation protein C or its cofactor - protein S );
- for thromboembolic arterial diseases , including angina pectoris or myocardial infarction (both in the active stage and in cases where the disease was suffered in the recent past);
- in case of active liver , and also if the patient’s liver biochemical parameters ;
- with porphyrin disease ;
- if you are aware of individual intolerance to estradiol , dydrogesterone or auxiliary components of Femoston;
- children and adolescents under 18 years of age;
- during pregnancy (both established and suspected pregnancy);
- during lactation.
Contraindications and side effects
The initiation of treatment with Femoston must be accompanied by a preliminary comprehensive medical examination of the woman. Data is collected on the presence of a number of diseases in the anamnesis, as well as other conditions for which taking the drug is contraindicated.
The main contraindications include:
- hormone-dependent neoplasms of the reproductive organs or mammary glands;
- vaginal bleeding, the cause of which is not clear;
- arterial hypertension;
- pregnancy and breastfeeding;
- thrombosis;
- kidney and liver diseases;
- endometriosis, etc.
Often refusal of Femoston therapy is the occurrence of the following side effects:
- swelling;
- disruption of the gastrointestinal tract;
- headache;
- pain in the mammary glands and pelvic organs;
- weight change.
Side effects
The category of side effects that often occur in connection with the use of Femoston includes: pain (headaches, in the abdomen, in the pelvic area), nausea, migraine attacks, flatulence, leg cramps, increased sensitivity and/or tenderness of the mammary glands, metrorrhagia, the appearance of bloody vaginal bleeding after menopause, asthenia, decrease/increase in body weight.
With a frequency of 1/1000-1/100 during clinical studies, the following phenomena occurred:
- vaginal candidiasis;
- depression;
- an increase in the size of uterine fibroids ;
- change in libido ;
- increased nervousness;
- DVT, PE;
- dizziness;
- gallbladder disease ;
- backache;
- allergic reactions on the skin, accompanied by itching, hives , rashes;
- dysmenorrhea;
- ulcerative defects on the cervix;
- the appearance of cervical discharge;
- peripheral edema.
In rare cases (with a frequency of 1/10000-1/1000), drug therapy was accompanied by:
- intolerance to contact lenses;
- functional liver , which often manifest themselves in the form of asthenia , malaise, abdominal pain, jaundice ;
- increased curvature of the cornea;
- enlargement of the mammary glands;
- premenstrual tension syndrome.
In isolated cases, the drug can provoke the development of chorea , hemolytic anemia, stroke, myocardial infarction, vascular purpura, vomiting, erythema nodosum or multimorphic, melanopathy or chloasma (often persisting even after discontinuation of the drug), angioedema , hypersensitivity reactions, worsening of porphyrin disease .
In addition, in connection with treatment with estrogen-progestagen drugs, women sometimes develop neoplasms (benign, malignant or of unknown etiology), the size of progestogen-dependent tumors fibrocystic lesions of the mammary glands appear of triglycerides in the blood plasma and the concentration of thyroid hormones increase ; arterial hypertension , acute arterial , peripheral vascular disease, varicose , dyspepsia , pancreatitis (against the background of pre-existing hypertriglyceridermia), systemic lupus erythematosus , cystitis-like syndrome , urinary incontinence develop epilepsy worsens , signs of dementia .
Femoston 2 set tab.p.p.o. No. 28 44562
Description
Estradiol 1 mg tablets: round, biconvex, white film-coated, embossed “S” above the “6” on one side and embossed “379” on the other. Tablets 1 mg estradiol / 10 mg dydrogesterone: round, biconvex, gray film-coated, embossed “S” above the “6” on one side and embossed “379” on the other. The tablet core is white. Estradiol 2 mg tablets: round, biconvex, pink film-coated, embossed with an “S” above the “6” on one side and embossed with “379” on the other. The tablet core is white. Tablets 2 mg estradiol/10 mg dydrogesterone: round, biconvex, light yellow film-coated, embossed with an “S” above the “6” on one side and “379” on the other side. The core of the tablet is white. After oral administration, micronized estradiol is easily absorbed. Metabolized in the liver to estrone and estrone sulfate, which also undergoes hepatic biotransformation. Glucuronides of estrone and estradiol are excreted primarily in the urine. Dydrogesterone after oral administration is quickly absorbed from the gastrointestinal tract. Metabolized completely. The main metabolite is 20-dihydrodydrogesterone, present in urine mainly in the form of a glucuronic acid conjugate. Complete elimination of dydrogesterone occurs after 72 hours. Estradiol, an estrogen included in the drug Femoston®, is identical to endogenous human estradiol. Estradiol replenishes the deficiency of estrogen in the female body after menopause and provides effective treatment of psycho-emotional and vegetative menopausal symptoms: hot flashes, increased sweating, sleep disturbances, increased nervous excitability, dizziness, headache, involution of the skin and mucous membranes, especially the mucous membranes of the genitourinary system (dryness and irritation of the vaginal mucosa, pain during sexual intercourse). Hormone replacement therapy (HRT) with Femoston® prevents bone loss in the postmenopausal period caused by estrogen deficiency. Taking Femoston® leads to a change in the lipid profile towards a decrease in the level of total cholesterol and LDL and an increase in HDL. Dydrogesterone is a progestogen, effective when taken orally, which completely ensures the onset of the secretion phase in the endometrium, thereby reducing the risk of developing endometrial hyperplasia and/or carcinogenesis, which increases against the background of estrogens. Dydrogesterone does not have estrogenic, androgenic, anabolic or glucocorticosteroid activity. The combination of 1 mg estradiol with dydrogesterone is a modern low-dose HRT regimen. Before prescribing or resuming HRT, it is necessary to collect a complete medical and family history and conduct a general and gynecological examination in order to identify possible contraindications and conditions requiring precautions. During treatment with the drug, it is recommended to periodically examine women (the frequency and nature of the studies are determined individually). In addition, it is advisable to conduct breast examination and/or mammography in accordance with accepted standards, taking into account clinical indications. The use of estrogens may affect the results of the following laboratory tests: glucose tolerance testing, thyroid and liver function tests. Generally recognized risk factors for thrombosis and thromboembolism while taking HRT are a history of thromboembolic complications, severe forms of obesity (body mass index more than 30 kg/m2) and systemic lupus erythematosus. There is no generally accepted opinion regarding the role of varicose veins in the development of thromboembolism. The risk of developing deep vein thrombosis of the lower extremities may temporarily increase with prolonged immobilization, major trauma, or surgery. In cases where prolonged immobilization is necessary after surgery, temporary cessation of HRT should be considered 4–6 weeks before surgery. When deciding on HRT in patients with recurrent deep vein thrombosis or thromboembolism receiving anticoagulant treatment, the benefits and risks of HRT must be carefully assessed. If thrombosis develops after starting HRT, the drug should be discontinued. The patient should be informed of the need to consult a doctor if the following symptoms occur: painful swelling of the lower extremities, sudden loss of consciousness, dyspnea, blurred vision. There is data demonstrating a slight increase in the detection rate of breast cancer in women who received HRT for a long time (more than 10 years). The likelihood of being diagnosed with breast cancer increases with the duration of treatment and returns to normal 5 years after stopping HRT. Patients who have previously received HRT using only estrogen drugs should be especially carefully examined before starting treatment in order to identify possible endometrial hyperstimulation. Breakthrough uterine bleeding and mild menstrual-like bleeding may occur in the first months of treatment with the drug. If, despite dose adjustment, such bleeding does not stop, the drug should be discontinued until the cause of the bleeding is determined. If bleeding recurs after a period of amenorrhea or continues after discontinuation of treatment, its etiology should be determined. This may require an endometrial biopsy. Femoston® is not a contraceptive. Perimenopausal patients are advised to use non-hormonal contraceptives. It does not affect the ability to drive a car or use other mechanisms.
Compound
Film-coated tablets 1 set. 1 white tablet estradiol 1 mg 1 gray tablet estradiol 1 mg dydrogesterone 10 mg excipients: lactose monohydrate; hypromellose; corn starch; colloidal silicon dioxide; magnesium stearate; Opadry OY-1-7000 white (for white tablets only); Opadry OY-8243 gray (only for gray tablets) in blister 28 pcs. (14 white and 14 gray); in a cardboard pack there are 1, 3 or 10 blisters. Film-coated tablets 1 set. 1 pink tablet estradiol 2 mg 1 light yellow tablet estradiol 2 mg dydrogesterone 10 mg excipients: lactose monohydrate; hypromellose; corn starch; colloidal silicon dioxide; magnesium stearate; Opadry OY-6957 pink (only for pink tablets), Opadry OY-02B22764 yellow (only for light yellow tablets) in a blister of 28 pcs. (14 pink and 14 light yellow); in a cardboard pack there are 1, 3 or 10 blisters.
Application
HRT for disorders caused by natural or surgical menopause; prevention of postmenopausal osteoporosis. Contraindicated during pregnancy and breastfeeding. From the blood and lymphatic system: very rarely (<0.01%) - hemolytic anemia. From the nervous system: headache, migraine (in 1–10%); sometimes (0.1–1%) - dizziness, nervousness, depression, changes in libido; very rarely - chorea. From the cardiovascular system: sometimes - venous thromboembolism; very rarely - myocardial infarction. From the gastrointestinal tract: nausea, abdominal pain, flatulence; very rarely - vomiting. From the liver and biliary tract: sometimes - cholecystitis; rarely (0.01–0.1%) - impaired liver function, sometimes accompanied by asthenia, malaise, jaundice or abdominal pain. From the skin and subcutaneous fat: sometimes - allergic reactions, rash, urticaria, itching, peripheral edema; very rarely - chloasma, melasma, erythema multiforme, erythema nodosum, hemorrhagic purpura, angioedema. From the reproductive system and mammary glands: soreness of the mammary glands, breakthrough bleeding, pain in the pelvic area; sometimes - changes in cervical erosion, changes in secretion, dysmenorrhea; rarely - breast enlargement, premenstrual-like syndrome. Other: changes in body weight; sometimes - vaginal candidiasis, breast carcinoma, increase in leiomyoma size; rarely - intolerance to contact lenses, increased corneal curvature; very rarely - exacerbation of porphyria (<0.01%). Drugs that are inducers of microsomal liver enzymes (barbiturates, phenytoin, rifampicin, rifabutin, carbamazepine) can weaken the estrogenic effect of Femoston®. Ritonavir and nelfinavir, although known as inhibitors of microsomal metabolism, may act as inducers when taken concomitantly with steroid hormones. Herbal preparations containing St. John's wort can stimulate the exchange of estrogens and progestogens. The interactions of dydrogesterone with other drugs are unknown. The patient should inform the doctor about the medications she is currently taking or was taking before prescribing Femoston®. Orally, preferably at the same time of day, regardless of meals - 1 tablet. per day without a break. Femoston® 1/10 is taken according to the following scheme: in the first 14 days of a 28-day cycle, take 1 white tablet daily (from half the package with an arrow marked with the number “1”) containing 1 mg of estradiol, in the remaining 14 days - daily 1 gray tablet (from half the package with the arrow marked "2") containing 1 mg estradiol and 10 mg dydrogesterone. Femoston® 2/10 is taken according to the following regimen: in the first 14 days of a 28-day cycle, take 1 tablet daily. pink (from half of the package with an arrow marked with the number “1”) containing 2 mg estradiol, and in the remaining 14 days - 1 light yellow tablet daily (from half of the package with an arrow marked with the number “2”) containing 2 mg estradiol and 10 mg dydrogesterone. For patients whose menstruation has not stopped, it is recommended to begin treatment on the first day of the menstrual cycle (1st day of the onset of menstruation). For patients with irregular menstrual cycles, it is advisable to begin treatment after 10–14 days of monotherapy with a progestogen. Patients whose last menstruation was observed more than 1 year ago can begin treatment at any time. Symptoms: nausea, vomiting, drowsiness, dizziness. Treatment: symptomatic, established or suspected pregnancy; breastfeeding period; diagnosed or suspected breast cancer, history of breast cancer; diagnosed or suspected estrogen-dependent malignancies; vaginal bleeding of unknown etiology; previous idiopathic or confirmed venous thromboembolism (deep vein thrombosis, pulmonary embolism); active or recent arterial thromboembolism; acute liver diseases, as well as a history of liver diseases (until normalization of laboratory parameters of liver function); untreated endometrial hyperplasia; hypersensitivity to any of the components of the drug; porphyria. With caution - patients receiving HRT and having the following conditions (currently or in the past) should be under close medical supervision: uterine leiomyoma, endometriosis; history of thrombosis or its risk factors; risk factors for estrogen-dependent tumors (for example, breast cancer in the patient’s mother); arterial hypertension; benign liver tumor; diabetes; cholelithiasis; epilepsy; migraine or intense headache; history of endometrial hyperplasia; systemic lupus erythematosus; bronchial asthma; renal failure; otosclerosis. After consultation with the doctor, the drug should be discontinued in cases such as: the appearance of jaundice or deterioration of liver function; strong rise in blood pressure; newly diagnosed migraine-like attack; pregnancy; manifestation of any contraindication.
Possible product names
- Femoston 2 (2/10) tab.p.p.o. No. 28
- FEMOSTON 2 MG/ 10 MG TAB. No. 28
- (Femoston) Femoston 2 (2/10) tab.p.p.o. No. 28
Femoston tablets, instructions for use
Most often, Femoston is taken on days strictly determined by the attending physician, taking into account the characteristics of a particular menstrual cycle . In the absence of menstrual bleeding, the tablets should be taken on the expected days when they should begin. With amenorrhea observed throughout the year, taking the drug can be started at any time.
Instructions for use Femoston 1/5
The drug is intended for continuous use: tablets are taken p/os, one per day (optimally at the same time), without reference to meal times. The duration of one cycle is 4 full weeks (1 package No. 28 is designed for one cycle). There is no need to take a break between cycles.
To relieve the symptoms of menopause, the drug is started with the minimum effective dose. Treatment begins with the appointment of Femoston 1/5. Taking into account the time of onset of menopause, the severity of the accompanying symptoms and the effectiveness of therapy, adjustments can be made to the dosage regimen.
If it is necessary to switch from another drug containing estrogen and progestogen components for sequential (or cyclic) use, the patient should complete the full four-week course and only after that switch to treatment with Femoston 1/5 (reception can be started on any day). There is no break between cycles.
The regimen for using Femoston 1/5 Conti is similar to that described above.
Instructions for use Femoston 1/10
Femoston 1/10 tablets should be taken regardless of meal time. The estrogen contained in the drug is intended for continuous daily use during the first two weeks of the cycle.
The progestogen component is added in the last 14 days of each four-week course.
Treatment begins with taking white tablets according to the following scheme: 1 tablet 1 time per day (at the same time) during the first 2 weeks of the cycle. Next, following the instructions on the package, they begin to take gray tablets (also, one per day).
There is no need to take breaks between 28-day cycles.
Sequential combined HRT begins with the prescription of Femoston 1/10, and then, if necessary, the dose is adjusted taking into account the clinical results of therapy.
To switch from a similar drug, you should complete the full cycle of treatment and only then start taking Femoston 1/10 tablets. You can do this any day.
Instructions for use Femoston 2/10
The estrogen component of the drug should be taken continuously, the progestogen component is administered from the 15th day of the 28-day cycle.
This means that in the first 2 weeks of the cycle the patient should take 1 pink tablet per day, and starting from the 15th day, following the instructions on the drug packaging, switch to taking yellow tablets.
Usually the starting dose of estradiol is 1 mg, so sequential combined HRT begins with Femoston 1/10 and, if necessary, moves to a higher dose over time.
Switching from other drugs to Femoston 2/10 is carried out only after completing a full four-week cycle (on any day).
How to take Femoston correctly if you miss the next dose?
If a woman misses the next dose of the drug, the tablet should be taken as quickly as possible. If more than 12 hours have passed since the missed dose, then the course is continued by taking the next tablet from the package (you do not need to drink the missed one).
Taking a double dose to compensate for a missed dose is not advisable, since it is associated with an increased risk of breakthrough bleeding and the appearance of spotting vaginal discharge.
How should patients of different age groups take the drug?
There is no sufficient experience with the use of Femoston for the treatment of patients over 65 years of age.
There are no indications for prescribing the drug to children and adolescents.
special instructions
The prescription of Femoston 1/10 is indicated only in the presence of symptoms that have an adverse effect on the quality of life. HRT is recommended until the risk of side effects exceeds the benefits of taking the drug. The limited clinical experience with the drug in women over 65 years of age should be taken into account.
In younger women, the absolute risk from using the drug is much lower than in older women.
To identify possible contraindications, the doctor should prescribe Femoston 1/10 based on a complete medical and family history and after a general gynecological examination of the patient, including mammography. The doctor should inform the woman about those changes in the mammary glands, the appearance of which requires consultation with a doctor. The use of the drug requires mandatory periodic examinations, at least once every 6 months. The doctor determines their nature and frequency individually.
The use of estrogens significantly increases the risk of developing endometrial cancer and hyperplasia, the degree of risk depends on the dose of the drug and the period of HRT. The combined composition of Femoston 1/10, namely cyclic administration of progestogen, reduces the risk of endometrial hyperplasia and cancer caused by estrogens. For timely diagnosis of these pathologies, it is advisable to conduct ultrasound screening, and, if necessary, histological examination. Bloody vaginal discharge, including breakthrough bleeding, may occur during the first months of therapy. If such bleeding occurs at later stages of treatment or occurs after discontinuation of the drug, it is necessary to diagnose their cause. To exclude malignancy, an endometrial biopsy is recommended.
The risk of developing deep vein thrombosis and pulmonary embolism during HRT increases several times, to a greater extent during the first 12 months of drug use. Patients whose first-degree relatives had thromboembolic complications at a young age, or with a history of spontaneous abortion, require a hemostasis study before prescribing the drug. With concomitant anticoagulant therapy, it is necessary to carefully evaluate the feasibility of prescribing Femoston 1/10. For planned surgery followed by long-term immobilization, it is recommended to discontinue HRT 1–1.5 months in advance and resume it only after the patient’s mobility has been completely restored. A woman should be informed about the symptoms of thromboembolism, such as shortness of breath, swelling or tenderness of the lower extremities, sudden chest pain, and the need to immediately consult a doctor if they occur.
The risk of developing breast cancer during combined estrogen-progestogen HRT, which lasts for more than 5 years, increases by 2 times. Breast engorgement caused by the drug may make it difficult to diagnose breast cancer in a timely manner.
There is a risk of developing ovarian cancer, but to a much lesser extent than breast cancer.
Combination therapy with estrogen and progestogen causes an increase in the relative risk of ischemic stroke, which is independent of the patient's age or duration of therapy. It should be borne in mind that the older the patient is at which she begins HRT, the higher the initial risk of ischemic stroke. Femoston 1/10 does not affect the occurrence of hemorrhagic stroke. The risk of developing coronary heart disease increases with age, but this can occur for objective and subjective reasons.
Femoston 1/10 is not a contraceptive.
In patients with impaired renal and cardiac function, their condition may be aggravated by the ability of estrogens to cause fluid retention.
In case of hypertriglyceridemia, HRT in very rare cases can contribute to the development of pancreatitis due to a significant increase in the level of triglyceride concentration in the blood plasma.
The use of the drug does not improve the patient's cognitive functions. When starting HRT after the age of 65, women have an increased risk of developing dementia.
Impact on the ability to drive vehicles and complex mechanisms
Due to the risk of unwanted reactions from the nervous system, it is recommended to exercise caution when operating complex machinery and vehicles.
Overdose
Cases of overdose with Femoston have not been recorded.
Both estrogen and progestogen components of the tablets belong to the category of low-toxic substances.
Theoretically, an overdose can provoke an increase in the severity of side effects such as nausea, vomiting, dizziness, drowsiness.
It is unlikely that an overdose may require any specific symptomatic treatment (including overdose in children).
Interaction
Drug interaction studies with Femoston have not been conducted.
However, it is known that some drugs may affect the effectiveness of estrogens and progesterones .
Thus, anticonvulsants (for example, phenytoin or phenobarbital ) and antimicrobial (including nevirapine , rifampicin or efavirenz enzymes of the cytochrome P450 system involved in drug metabolism .
Ritonavir and nelvinavir , which are potent inhibitors of CYP 3A4, A5 and A7 isoenzymes, in combination with steroid hormones , promote the activation of these cytochromes.
Herbal remedies based on St. John's wort (Hypericum perforatum) can stimulate the biotransformation of estrogens and progestogens due to the ability to influence the CYP 3A4 isoenzyme.
There is evidence that the more active metabolism of estrogens and progestogens provokes a decrease in the clinical effectiveness of these substances and affects the profile of uterine bleeding.
In turn, estrogens can disrupt the process of biotransformation of other substances due to competitive suppression of cytochromes of the P450 system , which take part in the processes of biotransformation of active drug substances.
This should be remembered when prescribing estrogens in combination with drugs that have a narrow therapeutic index, including fentanyl , tacrolimus , theophylline , cyclosporine .
Such combinations can cause an increase in the plasma concentration of these substances to a toxic level. Therefore, there may be a need for careful monitoring of the drug over an extended period of time, as well as a reduction in the dose of cyclosporine, tacrolimus, theophylline and fentanyl .
Analogs
Level 4 ATX code matches:
Klimonorm
The generic (structural analogue) of Femoston ⅕ is the drug Femoston Conti 1/5.
Drugs with a similar mechanism of action: Divina , Klimonorm , Kliogest , Trisequence .
Klimonorm or Femoston - which is better?
The decision about which drug from the group of combined estrogen-gestagen agents should be chosen is made by the doctor based on the data received from the patient about the period of age-related hormonal changes.
It is believed that in the drug Klimonorm the progestin component is present in the most optimal concentration, which allows effective control of the cycle and provides the necessary level of protection of the endometrium from the hyperplastic effect of estrogens .
At the same time, it is possible to maintain the beneficial effects caused by the influence of estrogens on the state of the cardiovascular system and lipid metabolism . In addition, levonorgestrel Klimonorm potentiates the effect of estradiol , aimed at the treatment and prevention of osteoporosis .
Another important feature of levonorgestrel is its almost 100% bioavailability, thanks to which it is possible to maintain the stability of the drug’s effects.
Moreover, the severity of the effects remains unchanged regardless of the woman’s nutritional characteristics, the presence of diseases of the digestive tract , as well as the activity of the liver system , which plays a key role in the processes of first-pass metabolism of xenobiotics .
The bioavailability of dydrogesterone , which is part of Femoston, is 28%, and therefore its effects are subject to fluctuations (both inter- and inter-individual).
Angelique or Femoston - which is better?
Experts believe that there is not much difference between these means. The main difference between the drug Angelique and Femoston is that it contains drospirenone progestational .
Which is better: Femoston, Klimonorm or Angelique?
Drugs of similar composition for the correction of menopausal symptoms include Klimonorm and Angeliq.
Just like Femoston, these are combined (estrogen and gestagen) agents. The main difference between the drug Angelique is its monophasic nature and the presence of a progestin component in the form of drospirenone. The main indications for use of the drug are menopause and the prevention of osteoporosis associated with a lack of intrinsic estrogen.
The drug is available in the form of tablets 28 and 84 pcs. with constant dosage:
- estradiol 1 mg;
- drospirenone 2 mg.
Klimonorm differs from previous drugs in the dosage of the active ingredients. The package contains:
- 9 estradiol tablets with a dosage of 2 mg;
- 12 combination pills (estradiol 2 mg, levonorgestrel 150 mcg).
Thus, one cycle involves taking 21 tablets once a day and 7 days off. Thanks to this feature, the drug is used not only in eliminating the symptoms of menopause, but also for correcting the menstrual cycle in women of reproductive age.
Consider whether it is worth taking it: side effects from using Duphaston.
Reviews of doctors and patients about Femoston tablets can be read at our link.
How to determine if you have hepatitis C: https://venerolog-ginekolog.ru/venereology/zppp/lechenie-gepatita-c.html
During pregnancy
The use of Femoston is contraindicated if it is known for sure that the woman is pregnant, as well as if there is reason to suspect pregnancy. The drug is also contraindicated for women who are breastfeeding.
In some cases, the medicine is prescribed during pregnancy planning. The indications are:
- conditions caused by estrogen and manifested by insufficiency of the first phase (that is, conditions in which by the end of the first (follicular) phase of the menstrual cycle the thickness of the endometrial layer does not exceed 7-8 mm);
- infertility caused by hormonal imbalance.
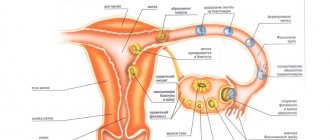
Too thin an endometrium can cause disruption of the luteal phase and, as a result, a woman cannot become pregnant.
Most often, at the planning stage, doctors recommend taking Femoston 2/10.
The concentration of estradiol in tablets intended for use during the first 2 weeks of the cycle is such that the drug, unlike contraceptives, does not suppress ovulation , while simulating the first phase of the menstrual cycle and stimulating the division and growth of endometrial .
Taking tablets containing estradiol supplemented with dydrogesterone, in turn, ensures secretory transformation of the inner layer of the uterus , which is necessary for normal implantation of the egg in the event of its fertilization and pregnancy. Thus, Femoston 2/10 allows you to normalize the disrupted menstrual cycle .
When planning pregnancy, Femoston 2/10 is taken from the first day of the menstrual cycle, one tablet per day for 4 full weeks. You should not stop treatment before the entire package is completed, as this can provoke a hormonal imbalance, manifested by breakthrough bleeding of varying degrees of intensity and leaving no chance of pregnancy.
Women who take Femoston when planning a pregnancy should further strengthen the luteal (second) phase of the cycle, therefore, from the 14th day of treatment, the patient is prescribed to take the drug in combination with Duphaston (or its analogue).
Dydrogesterone is present as gestagenic component in Duphaston , and this makes it possible to enhance the positive effect of therapy on the female body and the condition of the endometrium .
Duphaston is taken one tablet twice a day for a full two weeks.
Is it possible to get pregnant while taking the drug?
Pregnancy that occurs during the use of Femoston is an exception. As a rule, the chances of becoming pregnant after taking the drug for several cycles are considered more realistic, and this usually occurs after stopping treatment.
In extremely rare cases, it is possible to use the product against the background of an already existing pregnancy, when a woman needs endometrial . However, such a decision can only be made by a qualified specialist.
Are there any other analogs of Femoston?
Often, women during menopause, in addition to hormone-containing drugs, are also prescribed drugs of a different chemical nature. Main groups:
- homeopathic phytoestrogens (Remens, Klimadinon, Estrovel);
- antidepressants (Venlafaxine, Fluoxetine);
- antiepileptic drugs (Gabapentin).
For health reasons, other means of symptomatic therapy may be prescribed.
Hormonal antimenopausal drugs have a number of desirable and undesirable features associated with the effect of estrogens on the body. Before starting to take this group of drugs, you should always weigh all the advantages and disadvantages of the therapy.
See also a video about the use of Femoston during menopause:
Femoston reviews
A considerable number of reviews about Femoston 1/5 Conti have been left on the forums. Like reviews of Femoston 2/10 or 1/10, they are quite contradictory. As a rule, in reviews, women describe their experience of using the product during menopause or when planning pregnancy .
Those who were satisfied with the treatment note as the advantages of the drug that it is quite well tolerated and rarely causes side effects, quickly normalizes the condition, relieving the unpleasant symptoms of the onset of menopause , and improves overall well-being, has a positive effect on the condition of the skin, and restores the cycle in case of its violations, easy to use.
Negative reviews are associated with the occurrence of undesirable side effects (depression, rash, excess weight, swelling, decreased activity, joint pain, etc.), as well as the lack of the expected effect.
Turning to doctors' reviews of Femoston 1/10, 2/10 or 1/5, which are based on the results of clinical studies, we can conclude that the drug is a highly effective remedy for the treatment and prevention of conditions that have developed as a result of premature ovarian .
Moreover, all patients showed good tolerability of the tablets. Studies have made it possible to establish a pronounced positive effect of therapy on the general well-being of women and, in particular, on the blood lipid profile .
Against the background of the treatment, a significant increase in the rate of maximum oxygen consumption and an increase in the bone-protective effect of the estrogen component of Femoston by dydrogesterone were also established.
Thus, doctors confirm the need for early initiation and differentiated choice of hormone replacement therapy in women with “switched off” ovarian function .
Femoston and infertility
In addition to clinically proven indications for use, there is a practice of taking the drug in the treatment of infertility caused by hypofunction of the uterine epithelium. The prescription of Femoston when planning pregnancy is based on its ability to cause active growth of the endometrium in the first phase of the cycle. For a lasting therapeutic effect, it is recommended to continue therapy for 2-3 months. Pregnancy becomes possible after discontinuation of the drug.
This treatment tactic has both its supporters and opponents. Negative reviews from doctors and patients are usually associated with a high risk of side effects and the presence of a number of contraindications. However, the effect of the therapy is controversial.
Femoston price, where to buy
Price Femoston 1/5 in Russian pharmacies from 870 rubles. You can buy Femoston Conti 1/5 for an average of 900 rubles. Price Femoston 2/10 - from 790, Femoston 1/10 - from 795 rubles.
In Ukraine, the price of Femoston 1/10 is from 438 UAH, the price of Femoston 2/10 is from 520 UAH. The cost of the drug with a dosage of 1 mg + 5 mg is from 445 UAH.
- Online pharmacies in RussiaRussia
- Online pharmacies in UkraineUkraine
- Online pharmacies in KazakhstanKazakhstan
ZdravCity
- Femoston Mini tablets p.p.o.
2.5 mg+0.5 mg 28 pcs. Abbott Biologicals B.V. RUB 1,095 order - Femoston 2 tablets p.p.o. 2mg+10mg 28 pcs. Abbott Biologicals B.V.
RUR 1,021 order
- Femoston 1/5 conti tablets p.p.o. 28 pcs. Abbott Biologicals B.V.
1080 rub. order
- Femoston 1 tab. p/o captivity. 1mg+10mg No. 28 Abbott Biologicals B.V.
RUB 1,046 order
Pharmacy Dialogue
- Femoston 1 tablet No. 28Abbot
RUB 1,071 order
- Femoston conti tablets 1mg/5mg No. 28Abbot Biologicals
RUB 1,113 order
- Femoston 2 tablets No. 28Abbot
RUB 1,063 order
- Femoston mini tablets 2.5mg+0.5mg No. 28Abbot
RUB 1,063 order
show more
Pharmacy24
- Femoston Conti 1mg+5 mg No. 28 tablets Abbott Biologicals B.V., Netherlands
444 UAH.order - Femoston 1 mg/10 mg No. 56 tablets Abbott Biologicals B.V., Netherlands
816 UAH order
- Femoston Conti Mini 0.5 mg/2.5 mg No. 28 tablets Abbott Biologicals B.V., Netherlands
428 UAH. order
- Femoston 1mg+10mg No. 28 tablets Abbott Biologicals B.V., Netherlands
439 UAH. order
- Femoston 2 mg+10 mg No. 28 tablets Abbott Biologicals B.V., Netherlands
448 UAH order
PaniPharmacy
- Femoston tablets Femoston tablets 2mg+10mg No. 28 Netherlands, Solvay Biologicals
470 UAH. order
- Femoston conti tablets Femoston conti tablets 1mg+5mg No. 28 Netherlands, Solvay Biologicals
534 UAH. order
- Femoston tablets Femoston tablets 1mg+10mg No. 28 Netherlands, Abbott Biologicals
433 UAH order
- Femoston tablets Femoston tablets 1mg+10mg No. 56 Netherlands, Abbott Biologicals
795 UAH. order
show more