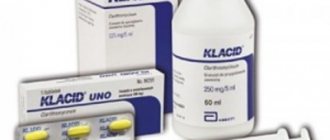
Klacid belongs to a group of drugs such as antibiotics and macrolites. This drug is available in several forms: powder, tablets and lyophilisate (a method of soft drying of substances in which the drug is dried by freezing). The drug contains granules with which you can prepare a suspension, capsules, and so on. To familiarize yourself with the indications for use, please use the instructions or information below.
Release form and composition
The main active component of the drug is the antibiotic clarithromycin.
The drug is produced in the following forms:
- Klacid 250 mg in immediate release tablet form. Each tablet is yellow in color and oval in shape.
- Klacid tablets, 500 mg of active ingredient, immediate release. The color is pale yellow.
- Long-acting Klacid SR tablets contain 500 mg.
- Suspension Klacid. It is produced in the form of a powder for dilution. Granular powder, white. Available in 125 and 250 mg, and packaged in 60 and 100 ml bottles. 5 ml of oral solution will contain 125 and 250 mg of clarithromycin, respectively. Klacid for children is used exclusively in the form of a suspension. Has a pleasant fruity smell
- In the form of a lyophilisate for intravenous administration. It has a white color and a light pleasant smell. One bottle contains 500 mg of active ingredient.
Storage conditions and shelf life
It is recommended that the sealed powder for suspension be stored at room temperature out of sight of small children for the entire shelf life of 2 years. After mixing with water, the drug can be stored at temperatures from +15 to +30 degrees for no longer than 14 days. It is not recommended to keep the medication in the refrigerator, and if it is not used within two weeks, it should be thrown away.
Injection tablets and vials should be kept in a place protected from sunlight, where the temperature does not exceed 25 degrees Celsius. The shelf life of the tablet form is 5 years, the injection form is 4 years.
Pharmacological characteristics
Klacid is a semi-synthetic antibiotic from the macrolide group. Under the influence of the main active ingredient of the drug, clarithromycin, the ability of pathogenic microorganisms to reproduce is suppressed by blocking bacterial protein synthesis.
The drug is highly effective against gram-positive and gram-negative flora, anaerobes, and some pathogens of sexually transmitted infections. Enterobacteriaceae and Pseudomonas aeruginosa show resistance to the active component of the drug.
When taking the tablet orally, the active substance of the drug is evenly distributed in the tissues of internal organs and biological fluids.
Pharmalogical action of the drug
As mentioned earlier, “Klacid” is an antibiotic that is classified as macrolites. "Klacid" has very high performance, which tests have shown. It has also shown high effectiveness in treating diseases such as legionnaires' disease. You can also notice very good effectiveness against pneumonia. Since “Klacid” is an antibiotic, it has antibacterial properties, so it can effectively fight against Staphylococcus aureus, as well as other diseases.
Why is Klacid prescribed?
Before starting therapy with Klacid, it is important to conduct a sensitivity study of pathogens.
The following indications for use are registered for Klacid:
- diseases of the upper and lower respiratory tract (pharyngitis, tonsillitis, pneumonia, sinusitis, tonsillitis, tracheitis, sinusitis, bronchitis, whooping cough);
- diseases accompanied by infectious lesions of soft tissues (wounds, boils, purulent acne (pimples), etc.);
- infectious lesions of the ENT organs (otitis media);
- pathologies caused by Mycobacteria, especially in patients with HIV infection.
Indications
The medicine is prescribed:
- with bronchitis;
- with pharyngitis;
- with pneumonia;
- for sinusitis and other sinusitis;
- with otitis media;
- with laryngitis;
- with tracheitis;
- for folliculitis, impetigo, erysipelas and other skin lesions;
- with mycobacterial infection;
- when infected with chlamydia;
- to eliminate H. pylori and prevent relapses of peptic ulcer;
- for pulpitis, periodontitis and other infections called odontogenic.
The medication is recommended to be used only when infected with microorganisms that are sensitive to clarithromycin. When prescribing medicine for sore throat, whooping cough, scarlet fever and other infectious diseases, it is first advisable to determine whether Klacid will work on their causative agent.
Medical instructions for use
Klacid tablets are intended to be taken orally, regardless of food. It is recommended to swallow the tablet immediately with the required amount of water. The dosage of the drug largely depends on the severity of the disease and the individual characteristics of the body.
For inflammatory diseases that are not accompanied by any complications, the daily dose of the drug is 500 mg - 1 tablet in the morning, or 1 tablet in the morning and evening with a dosage of 250 mg.
If there are complications, it is possible to increase the daily dose to 1 g under the supervision of a physician. The course of treatment with the drug usually does not exceed 5-7 days; if there is no expected effect or the patient’s condition worsens, you should contact a specialist again; perhaps the infectious agents are resistant to the drug or the diagnosis was initially made incorrectly.
Contraindications for use
- Breastfeeding period;
- Hypokalemia (risk of QT prolongation);
- Severe liver failure due to renal failure;
- Hypersensitivity to the components of the drug and other macrolides;
- A history of ventricular arrhythmia or ventricular tachycardia of the “pirouette” type, prolongation of the QT interval;
- A history of cholestatic hepatitis or jaundice that developed during the use of clarithromycin;
- Concomitant use with cisapride, pimozide, astemizole, terfenadine; ergotamine, dihydroergotamine and other ergot alkaloids; midazolam for oral administration; colchicine;
- Combination with HMG-CoA reductase inhibitors (statins), which are mainly metabolized by the CYP3A4 isoenzyme (simvastatin, lovastatin), due to the high risk of developing myopathy and rhabdomyolysis.
Side effects
If Klacid is administered IV or taken orally, a number of side effects may occur. If such effects occur after intravenous administration or ingestion of tablets, you must inform your specialist. The following manifestations are possible:
- Functions of the central nervous system: changes in taste, headaches.
- Digestive system: nausea, abdominal pain, diarrhea, vomiting, dyspepsia.
- Local reactions when injecting the solution: inflammatory processes at the injection site, phlebitis, pain during palpation.
- Laboratory indicators: increased activity of liver enzymes.
In addition to these side effects, there are possible side effects that occur less frequently:
- oral candidiasis;
- thrombocytopenia, leukopenia;
- anaphylactic reactions;
- hypoglycemia;
- mental disorders, insomnia;
- dizziness, convulsions;
- myalgia;
- reversible hearing loss;
- ventricular tachycardia;
- stomatitis, acute pancreatitis, glossitis;
- liver dysfunction;
- hives, rash;
- increased creatinine levels in the blood.
Use during pregnancy and lactation
In the first trimester of pregnancy, the use of Klacid tablets is contraindicated, as this can cause severe damage to the fetus in the womb and lead to congenital abnormalities.
In the second and third trimesters of pregnancy, drug therapy is carried out only for strict health reasons, in the case when the expected benefit for the woman exceeds the likely risks for the unborn baby.
During breastfeeding, treatment with the drug is not carried out, since clarithromycin is excreted in breast milk. If therapy is necessary, a woman should stop lactation.
How to take it for children?
Klacid for children can be used from the age of three. In most cases, children are prescribed Klacid suspension. Reviews for children indicate that this drug is quite effective. At the same time, the price of the suspension is quite high. The dosage for children is as follows: 7.5 mg per 1 kg of child’s weight twice a day. The highest daily dose is 500 mg.
Children over 12 years of age are prescribed 250 mg (tablets) twice a day. There is evidence that children tolerate Klacid more easily than other antibiotics. Therefore, the drug is often prescribed for sore throat, bronchitis, pneumonia, etc. However, we should not forget that side effects still occur.
Analogs
- Arvicin;
- Arvicin retard;
- Binoculars;
- Zimbaktar;
- Kispar;
- Clubax;
- Clarbuckt;
- Clarithromycin;
- Clarithrosin;
- Claricin;
- Claricite;
- Claromine;
- Klasine;
- Klacid SR;
- Clerimed;
- Coater;
- Crixan;
- Seydon-Sanovel;
- CP-Klaren;
- Fromilid;
- Fromilid Uno;
- Ecositrin.
When choosing analogues, you must remember that the instructions for use of Klacid, price and reviews do not apply to drugs of similar effect. Replacing the drug is permissible only after the recommendation of a doctor.
The differences between Klacid and Klacid SR are that the latter drug is a long-acting drug, that is, the active substance is released more slowly.
Klacid for pneumonia in children
Every year, with the constant emergence of a variety of new drugs, harmful microorganisms change, adapt and become increasingly resistant to drugs. Today, it is very common to find bacteria that do not respond to the effects of antibiotics of the erythromycin and penicillin series.
For this reason, the pharmacological industry is almost constantly working to create an increasing number of different types of new generation antibiotics, which are synthetic and semi-synthetic improved derivatives of the most well-known antimicrobial and antibacterial agents.
Klacidus also belongs to this category.
- Release forms and composition
- Analogs
- When treating children
- During pregnancy and lactation
special instructions
Clarithromycin is eliminated primarily by the liver. In this regard, caution should be exercised when prescribing Klacid to patients with impaired liver function.
- In the presence of chronic liver diseases, it is necessary to regularly monitor serum enzymes.
- Caution should be observed when treating patients with moderate and severe renal failure with Klacid.
- In clinical practice, cases of toxicity of colchicine when combined with clarithromycin have been described, especially in elderly people. Some of them were observed in patients with renal failure; Several deaths have been reported in similar patients.
- The possibility of cross-resistance between clarithromycin and other macrolide drugs, as well as lincomycin and clindamycin, must be considered.
Prescribe with caution against drugs metabolized by the liver.
In case of co-administration with warfarin or other indirect anticoagulants, it is necessary to monitor the prothrombin time.
Drug interactions
It is strictly forbidden to take an antibiotic with the following medications: astemizole, cisapride, pimozide, terfenadine. Combining these drugs with clarithromycin can cause serious side effects on the heart. For example, arrhythmia, ventricular fibrillation or ventricular tachycardia.
Do not take with ergot alkaloids; simultaneous use with ergotamine can provoke the development of poisoning. Poor compatibility with rifabutin, as it reduces the concentration of the antibacterial drug in the blood.
Rifapentine, etravirine, neviparine or efavirenz may reduce the effectiveness of treatment by reducing plasma concentrations. Along with ritonavir, dosage adjustment is necessary; how much and in what quantities to take the medicine will be indicated by the attending physician.
With the use of insulin, it is necessary to regularly measure glucose readings, since the drug can provoke hypoglycemia. With quinidine, there is a chance of ventricular tachycardia with further transition to fibrillation. It is not advisable to use it together with warfarin and simvastatin. During therapy with sildanefil or tadafil, the dose of stimulants should be reduced.
Theophylline and carbamazepine increase the concentration of drugs in the blood. There is a risk of drowsiness and delirium with triazolam. With drugs, the concentration of digoxin in the blood increases. Diacordin and verpamil help lower blood pressure when used simultaneously with drugs.
Klacid®
The use of the following drugs with clarithromycin is contraindicated due to the potential for serious side effects:
Cisapride and pimozide
When used together, it is possible to increase the concentration of cisapride/pimozide in the blood plasma, increase the QT interval, and cause cardiac arrhythmias, including ventricular tachycardia, ventricular fibrillation and torsade de pointes (TdP).
Terfenadine and astemizole
When used together, it is possible to increase the concentration of terfenadine/astemizole in the blood, increase the QT interval, and cause cardiac arrhythmias, including ventricular tachycardia, ventricular fibrillation and torsades de pointes.
Ergotamine/dihydroergotamine
When used together, the following effects associated with acute poisoning with drugs from the ergotamine group are possible: vascular spasm, ischemia of the limbs and other tissues, including the central nervous system.
Effect of other drugs on clarithromycin
Drugs that are CYP3A inducers (for example, rifampicin, phenytoin, carbamazepine, phenobarbital, St. John's wort) may induce the metabolism of clarithromycin. This may result in subtherapeutic concentrations of clarithromycin, resulting in reduced effectiveness. In addition, it is necessary to monitor the concentration of CYP3A inducer in the blood plasma, which may increase due to inhibition of CYP3A by clarithromycin. When rifabutin and clarithromycin were used together, an increase in plasma concentrations of rifabutin and a decrease in serum concentrations of clarithromycin were observed with an increased risk of developing uveitis.
The following drugs have a proven or suspected effect on clarithromycin plasma concentrations; if used concomitantly with clarithromycin, dosage adjustments or switching to alternative treatment may be required.
Efavirenz, nevirapine, rifampicin, rifabutin and rifapentine
Strong inducers of the cytochrome P450 system, such as efavirenz, nevirapine, rifampicin, rifabutin and rifapentine, can accelerate the metabolism of clarithromycin and, thus, reduce the plasma concentration of clarithromycin and weaken the therapeutic effect, and at the same time increase the concentration of 14-OH-clarithromycin, a metabolite. also being microbiologically active. Since the microbiological activity of clarithromycin and 14-OH-clarithromycin differs against different bacteria, the therapeutic effect may be reduced when clarithromycin is used together with enzyme inducers.
Etravirine
The concentration of clarithromycin decreases with the use of etravirine, but the concentration of the active metabolite 14-OH-clarithromycin increases. Because 14-OH-clarithromycin has low activity against MAC infections, overall activity against MAC infections may be affected, and alternative treatments should be considered for the treatment of MAC.
Fluconazole
Coadministration of fluconazole 200 mg daily and clarithromycin 500 mg twice daily in 21 healthy volunteers resulted in an increase in mean clarithromycin minimum steady-state concentration (Cmin) and AUC by 33% and 18%, respectively. However, co-administration did not significantly affect the average steady-state concentration of the active metabolite 14-OH-clarithromycin. No dose adjustment of clarithromycin is required when taking fluconazole concomitantly.
Ritonavir
A pharmacokinetic study showed that co-administration of ritonavir 200 mg every 8 hours and clarithromycin 500 mg every 12 hours resulted in a marked suppression of the metabolism of clarithromycin. When coadministered with ritonavir, clarithromycin Cmax increased by 31%, Cmin increased by 182%, and AUC increased by 77%. Complete suppression of the formation of 14-OH-clarithromycin was noted. Due to the wide therapeutic range of clarithromycin, dose reduction is not required in patients with normal renal function. In patients with renal failure, it is advisable to consider the following dose adjustment options: with CC 30-60 ml/min, the dose of clarithromycin should be reduced by 50%; with CC less than 30 ml/min, the dose of clarithromycin should be reduced by 75%. Ritonavir should not be co-administered with clarithromycin in doses exceeding 1 g/day.
Oral hypoglycemic agents/insulin
When clarithromycin is used concomitantly with oral hypoglycemic agents and/or insulin, severe hypoglycemia may occur. During concomitant use of clarithromycin and certain drugs that lower glucose concentrations, such as nateglinide, pioglitazone, repaglinide and rosiglitazone, inhibition of the CYP3A isoenzyme by clarithromycin may occur, which may result in hypoglycemia. Careful monitoring of glucose concentrations is recommended.
Effect of clarithromycin on other drugs
Antiarrhythmic drugs (quinidine and disopyramide)
Ventricular tachycardia of the “pirouette” type may occur with the combined use of clarithromycin and quinidine or disopyramide. When clarithromycin is coadministered with these drugs, ECG monitoring should be performed regularly to monitor for QT interval prolongation, and serum concentrations of these drugs should also be monitored.
CYP3A-mediated interactions
Co-administration of clarithromycin, which is known to inhibit the CYP3A isoenzyme, and drugs primarily metabolized by CYP3A may be associated with a mutual increase in their concentrations, which may increase or prolong both therapeutic and side effects. Clarithromycin should be used with caution in patients receiving drugs that are substrates of the CYP3A isoenzyme, especially if these drugs have a narrow therapeutic index (for example, carbamazepine) and/or are extensively metabolized by this enzyme. If necessary, the dose of the drug taken together with clarithromycin should be adjusted. Also, whenever possible, serum concentrations of drugs primarily metabolized by CYP3A should be monitored. The following drugs/classes are metabolized by the same CYP3A isoenzyme that metabolizes clarithromycin, e.g., alprazolam, carbamazepine, cilostazol, cyclosporine, disopyramide, ergot alkaloids, methylprednisolone, midazolam, omeprazole, indirect anticoagulants (eg, warfarin), quinidine, rifabutin, sildenafil , tacrolimus, triazolam and vinblastine. Also CYP3A agonists include the following drugs that are contraindicated for combined use with clarithromycin: astemizole, cisapride, pimozide, terfenadine, lovastatin, simvastatin and ergot alkaloids. Drugs that interact in this manner through other isoenzymes within the cytochrome P450 system include phenytoin, theophylline, and valproic acid.
HMG-CoA reductase inhibitors
Like other macrolides, clarithromycin increases concentrations of HMG-CoA reductase inhibitors (eg, lovastatin and simvastatin). Co-administration of clarithromycin with lovastatin or simvastatin is contraindicated. Patients should be evaluated for signs and symptoms of myopathy. There are rare reports of the development of rhabdomyolysis in patients receiving therapy with atorvastatin or rosuvastatin in combination with clarithromycin. When combined with clarithromycin, atorvastatin or rosuvastatin should be used in the lowest possible doses or statins that are not dependent on CYP3A metabolism (for example, fluvastatin or pravastatin) should be used.
Oral anticoagulants
When taking warfarin and clarithromycin together, bleeding and a marked increase in MHO and prothrombin time are possible. In case of combined use with warfarin or other indirect anticoagulants, it is necessary to monitor MHO and prothrombin time.
Omeprazole
Clarithromycin (500 mg every 8 hours) was studied in healthy adult volunteers in combination with omeprazole (40 mg daily). When clarithromycin and omeprazole were co-administered, steady-state plasma concentrations of omeprazole were increased (Cmax, AUC0-24, and T1/2 increased by 30%, 89% and 34%, respectively). The average gastric pH over 24 hours was 5.2 when taking omeprazole alone and 5.7 when taking omeprazole with clarithromycin.
Sildenafil, tadalafil and vardenafil
Each of these phosphodiesterase inhibitors is metabolized at least in part by CYP3A. However, CYP3A may be inhibited in the presence of clarithromycin. Concomitant use of clarithromycin with sildenafil, tadalafil or vardenafil may result in increased phosphodiesterase inhibitory effects. When using these drugs together with clarithromycin, consider reducing the dose of sildenafil, tadalafil and vardenafil.
Theophylline, carbamazepine
When clarithromycin and theophylline or carbamazepine are used together, the concentration of these drugs in the systemic circulation may increase.
Tolterodine
The primary metabolism of tolterodine occurs through the 2D6 isoform of cytochrome P450 (CYP2D6). However, in a portion of the population lacking CYP2D6, metabolism occurs via CYP3A. In this population, inhibition of CYP3A results in significantly higher serum concentrations of tolterodine. In populations that are poor metabolizers via CYP2D6, a reduced dose of tolterodine may be required in the presence of CYP3A inhibitors such as clarithromycin.
Triazolobenzodiazepines (eg, alprazolam, midazolam, triazolam)
With the combined use of midazolam and clarithromycin in the form of tablets (500 mg 2 times / day), an increase in the AUC of midazolam was noted: 2.7 times after intravenous administration of midazolam and 7 times after oral administration. Concomitant oral administration of midazolam and clarithromycin should be avoided. If intravenous midazolam is used concomitantly with clarithromycin, the patient's condition should be carefully monitored for possible dose adjustment. The same precautions should be applied to other benzodiazepines that are metabolized by CYP3A, including triazolam and alprazolam. For benzodiazepines whose elimination is not dependent on CYP3A (temazepam, nitrazepam, lorazepam), a clinically significant interaction with clarithromycin is unlikely.
When clarithromycin and triazolam are used together, CNS effects such as drowsiness and confusion are possible. Therefore, if coadministration occurs, it is recommended to monitor for symptoms of CNS impairment.
Interaction with other drugs
Colchicine
Colchicine is a substrate of both CYP3A and the P-glycoprotein (Pgp) transporter protein. Clarithromycin and other macrolides are known to be inhibitors of CYP3A and Pgp. When clarithromycin and colchicine are taken together, inhibition of Pgp and/or CYP3A may result in increased effects of colchicine. The development of clinical symptoms of colchicine poisoning should be monitored. There have been post-marketing reports of cases of colchicine poisoning when taken concomitantly with clarithromycin, most often in elderly patients. Some of the reported cases occurred in patients with renal failure. Some cases were reported to be fatal.
Digoxin
Digoxin is thought to be a substrate for Pgp. Clarithromycin is known to inhibit Pgp. When clarithromycin and digoxin are co-administered, inhibition of Pgp by clarithromycin may result in increased effects of digoxin. Coadministration of digoxin and clarithromycin may also result in increased serum concentrations of digoxin. Some patients have experienced clinical symptoms of digoxin toxicity, including potentially fatal arrhythmias. Serum digoxin concentrations should be carefully monitored when clarithromycin and digoxin are coadministered.
Zidovudine
Concomitant use of oral clarithromycin tablets and zidovudine by adult HIV-infected patients may result in a decrease in the steady-state concentration of zidovudine. Because clarithromycin interferes with the oral absorption of zidovudine, the interaction can be largely avoided by taking clarithromycin and zidovudine 4 hours apart. This interaction has not been observed in HIV-infected children taking clarithromycin pediatric suspension with zidovudine or dideoxyinosine. Since clarithromycin may interfere with the absorption of zidovudine when administered concomitantly orally in adult patients, such an interaction is unlikely to occur when clarithromycin is used intravenously.
Phenytoin and valproic acid
There is evidence of interactions between CYP3A inhibitors (including clarithromycin) and drugs that are not metabolized by CYP3A (phenytoin and valproic acid). For these drugs, when used together with clarithromycin, it is recommended to determine their serum concentrations, as there are reports of their increase.
Bidirectional drug interactions
Atazanavir
Clarithromycin and atazanavir are both substrates and inhibitors of CYP3A. There is evidence of a bidirectional interaction between these drugs.
Co-administration of clarithromycin (500 mg twice daily) and atazanavir (400 mg once daily) may result in a twofold increase in clarithromycin exposure and a 70% decrease in 14-OH-clarithromycin exposure, with a 28% increase in atazanavir AUC. Due to the wide therapeutic range of clarithromycin, dose reduction is not required in patients with normal renal function. In patients with moderate renal failure (creatinine clearance 30-60 ml/min), the dose of clarithromycin should be reduced by 50%. In patients with CC less than 30 ml/min, the dose of clarithromycin should be reduced by 75% using the appropriate dosage form of clarithromycin. Clarithromycin in doses exceeding 1000 mg/day should not be used in combination with protease inhibitors.
Itraconazole
Clarithromycin and itraconazole are substrates and inhibitors of CYP3A, which determines the bidirectional interaction of the drugs. Clarithromycin may increase plasma concentrations of itraconazole, while itraconazole may increase plasma concentrations of clarithromycin. Patients taking itraconazole and clarithromycin concomitantly should be closely monitored for symptoms of increased or prolonged pharmacological effects of these drugs.
Saquinavir
Clarithromycin and saquinavir are CYP3A substrates and inhibitors, resulting in a bidirectional drug interaction. Coadministration of clarithromycin (500 mg twice daily) and saquinavir (soft gelatin capsules, 1200 mg three times daily) in 12 healthy volunteers increased the AUC and Cmax of saquinavir by 177% and 187%, respectively, compared with saquinavir alone. . The AUC and Cmax values of clarithromycin were approximately 40% higher than with clarithromycin monotherapy. When these two drugs are used together for a limited time at the doses/formulations indicated above, no dose adjustment is required. Results from drug interaction studies using saquinavir soft gelatin capsules may not be consistent with the effects observed with saquinavir hard gelatin capsules. The results of drug interaction studies with saquinavir monotherapy may not be consistent with the effects observed with saquinavir/ritonavir therapy. When taking saquinavir with ritonavir, consider the potential effect of ritonavir on clarithromycin .
Verapamil
When taken together with clarithromycin, arterial hypotension, bradyarrhythmia and lactic acidosis are possible.
What do the reviews say?
Reviews about Klacida are both positive and not too enthusiastic. Many patients note that the antibiotic is effective and significantly speeds up the healing process. But there are also stories that the drug provoked side effects, and as a result the doctor was forced to select a different antibiotic for treatment.
Parents who gave the drug to their children also leave different reviews of Klacid. No less important are the reviews of doctors, which indicate that experts consider this antibiotic to be effective and is often prescribed to both adults and children.
Manufacturer
One of the largest pharmaceutical companies, Abbott has operations throughout the world. Its factories and representative offices are located in many countries and continents. This nuance is associated with consumer confusion about the origin of Klacid. On different packages of the same drug, Germany, France, Belgium and other countries may be indicated as the manufacturer. Confused consumers sometimes accuse pharmacists of selling “counterfeits” and “counterfeits.” Let's understand the pharmaceutical tricks together.
In reality, Abbott is an American company that today operates in more than 150 countries around the world. It can confidently be called a pharmaceutical giant on the level of Pfizer. Created in 1888, the small enterprise has today become one of the largest drug manufacturers, whose specialists create new drugs.
Like many other large corporations, Abbott has dispersed production around the world, a technique that allows for some cost savings and a reduction in the final cost of the drug. That is why Klacid, which we are talking about today, can be released in France, and in Italy, and in many other countries.
The packaging of Klacida tablets does not indicate the location of the central office of the Abbott company, located in Illinois, USA, but the address of the plant where this series of the drug was created. Therefore, buyers should not be confused by changes in the country of origin.
>>We recommend: if you are interested in effective methods of getting rid of chronic runny nose, pharyngitis, tonsillitis, bronchitis and persistent colds, then be sure to check out this page of the site after reading this article. The information is based on the author’s personal experience and has helped many people, we hope it will help you too. Now let's return to the article.<<