pharmachologic effect
A drug for the treatment of benign prostatic hyperplasia (BPH). A competitive and specific inhibitor of type II 5-alpha reductase, an intracellular enzyme that converts testosterone into a more active androgen, dihydrotestosterone (DHT). It is a synthetic 4-azasteroid compound. Effectively reduces the level of DHT both in the blood and in the glandular tissue.
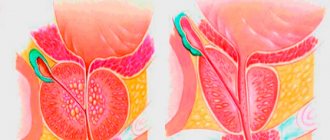
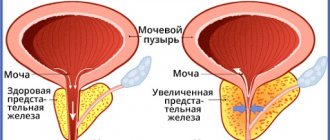
Suppression of DHT formation is accompanied by a decrease in the size of the prostate gland, an increase in the maximum flow rate of urine and a decrease in the severity of symptoms associated with prostatic hyperplasia.
Finasteride has no affinity for androgen receptors. The drug does not affect the lipid profile, as well as the levels of cortisol, estradiol, prolactin, thyroid-stimulating hormone and thyroxine in the blood plasma.
A single dose of finasteride at a dose of 5 mg leads to a rapid decrease in the concentration of DHT in the blood serum with maximum effect achieved after 8 hours.
When Proscar was used for 7-10 days in patients undergoing prostatectomy, there was an approximately 80% decrease in DHT levels in prostate tissue and an increase in testosterone levels in prostate tissue by 10 times compared to their pre-treatment levels.
It was found that long-term (over 4 years) use of Proscar in patients with BPH (with moderate or severe symptoms of the disease) reduced the risk of developing urological complications and surgical interventions (transurethral resection of the prostate or prostatectomy; acute urinary retention requiring catheterization) by 51% and was accompanied by a pronounced and persistent decrease in prostate volume, as well as a persistent increase in maximum urinary flow rate and improvement in symptoms (PLESS Study).
In patients who took Proscar for 3 months and achieved a reduction in prostate volume of approximately 20%, when treatment was stopped, the prostate volume returned to its previous size after 3 months.
Thus, Proscar causes a reduction in the size of an enlarged prostate gland, improves urine flow and reduces symptoms associated with BPH.
Pharmacodynamics and pharmacokinetics
Pharmacodynamics
Finasteride is a selective blocker of type 2 5-alpha reductase , an enzyme that transforms testosterone into dihydrotestosterone . With prostate adenoma, its growth depends on the conversion of testosterone to dihydrotestosterone in prostate cells. Proscar is highly effective in reducing the latter and has no affinity for androgen receptors.
Pharmacokinetics
After taking the drug, 39% of the dose is evacuated in the urine, another 57% in feces. The bioavailability of finasteride is 80%. The highest level in the blood is reached after about 2 hours. Absorption of the drug from the digestive tract is completed 7 hours after use. The half-life is on average 6 hours. Plasma protein binding is 93%. In elderly patients, the rate of elimination of the active substance is slightly reduced. Penetrates the blood-brain barrier.
Indications for use
Treatment of benign prostatic hyperplasia for the purpose of:
- prevention of urological complications (reducing the risk of acute urinary retention) and reducing the need for surgical operations (including transurethral resection of the prostate gland and prostatectomy);
- reducing the size of an enlarged prostate gland, improving urine flow and reducing the severity of symptoms associated with benign prostatic hyperplasia.
Therapy with Proscar is indicated for patients with an enlarged prostate gland.
Directions for use and doses
The recommended dose is 5 mg (1 tablet) 1 time/day, regardless of meals.
Proscar can be used as monotherapy or in combination with doxazosin.
For patients with renal failure of varying severity (with a decrease in CC to 9 ml/min), individual dose selection is not required, since pharmacokinetic studies have not revealed any changes in the distribution of finasteride in this category of patients.
There is also no need for dose adjustment in elderly patients, although pharmacokinetic studies indicate that the elimination of finasteride is slightly reduced in patients over 70 years of age.
Proscar
Release form, composition and packaging
Blue film-coated tablets; Apple-shaped, with "MSD 72" engraved on one side and "PROSCAR" on the other. 1 tab. finasteride 5 mg. Excipients: lactose monohydrate, pregelatinized starch (corn), sodium salt of starch carboxymethyl ether, yellow iron oxide, sodium docusate, microcrystalline cellulose, magnesium stearate. Film shell composition: methylhydroxypropylcellulose, hydroxypropylcellulose, titanium dioxide, talc, indigo carmine aluminum varnish.
Clinical and pharmacological group: Drug for the treatment of benign prostatic hyperplasia.
pharmachologic effect
A drug for the treatment of benign prostatic hyperplasia (BPH). A competitive and specific inhibitor of type II 5-alpha reductase, an intracellular enzyme that converts testosterone into a more active androgen, dihydrotestosterone (DHT). It is a synthetic 4-azasteroid compound. Effectively reduces the level of DHT both in the blood and in the glandular tissue. Suppression of DHT formation is accompanied by a decrease in the size of the prostate gland, an increase in the maximum flow rate of urine and a decrease in the severity of symptoms associated with prostatic hyperplasia.
Finasteride has no affinity for androgen receptors. The drug does not affect the lipid profile, as well as the levels of cortisol, estradiol, prolactin, thyroid-stimulating hormone and thyroxine in the blood plasma. A single dose of finasteride at a dose of 5 mg leads to a rapid decrease in the concentration of DHT in the blood serum with maximum effect achieved after 8 hours.
Although plasma levels of finasteride fluctuate over a 24-hour period, DHT levels remain constant. This means that the plasma concentration of finasteride is not directly related to the plasma concentration of DHT.
In patients with BPH treated with Proscar 5 mg/day for 4 years, there was an approximately 70% decrease in blood DHT concentrations and an approximately 20% decrease in prostate volume. In addition, prostate-specific antigen (PSA) levels were reduced by approximately 50% compared with baseline levels, suggesting a reduction in prostate epithelial cell growth. Testosterone levels in the blood increased by approximately 10-20%.
When Proscar was used for 7-10 days in patients undergoing prostatectomy, there was an approximately 80% decrease in DHT levels in prostate tissue and an increase in testosterone levels in prostate tissue by 10 times compared to their pre-treatment levels.
It was found that long-term (over 4 years) use of Proscar in patients with BPH (with moderate or severe symptoms of the disease) reduced the risk of developing urological complications and surgical interventions (transurethral resection of the prostate or prostatectomy; acute urinary retention requiring catheterization) by 51% and was accompanied by a pronounced and persistent decrease in prostate volume, as well as a persistent increase in maximum urinary flow rate and improvement in symptoms (PLESS Study).
In patients who took Proscar for 3 months and achieved a reduction in prostate volume of approximately 20%, when treatment was stopped, the prostate volume returned to its previous size after 3 months. Thus, Proscar causes a reduction in the size of an enlarged prostate gland, improves urine flow and reduces symptoms associated with BPH.
Pharmacokinetics
Suction
Absorption of the drug from the gastrointestinal tract ends 6-8 hours after administration. When taken orally, bioavailability is about 80%. Eating does not interfere with the bioavailability of the drug. Cmax of finasteride in blood plasma is achieved approximately 2 hours after oral administration.
Distribution
Plasma protein binding is 93%. Vd is 76 l. A study of repeated administration of finasteride in therapeutic doses over a long period of time showed the possibility of accumulation of the drug in the body in small quantities. With daily oral administration of finasteride at a dose of 5 mg/day, its plasma concentration reaches 8-10 ng/ml and remains at this level for a long time.
In patients who received Proscar for 7-10 days, finasteride penetrates the BBB, but the drug content in the cerebrospinal fluid does not reach significant concentrations. When taking Proscar at a dose of 5 mg/day, its penetration into the seminal fluid was also noted. At the same time, the concentration of finasteride in the seminal fluid of adult men was 50-100 times lower than its concentration in the blood plasma.
Metabolism and excretion
T1/2 of finasteride from the blood averages 6 hours. Systemic clearance is about 165 ml/min. In men, after oral administration of a single dose of finasteride, 39% of the dose taken was excreted in the urine in the form of metabolites (presumably unchanged finasteride is not excreted in the urine); 57% of the dose taken was excreted in the feces. It has been established that two metabolites of finasteride have a less pronounced inhibitory effect on 5-alpha reductase.
Pharmacokinetics in special clinical situations
In elderly patients, the rate of elimination of finasteride is slightly reduced. In men over 70 years of age, T1/2 of finasteride is about 8 hours, compared to individuals aged 18 to 60 years, in whom T1/2 of finasteride is 6 hours. However, this is not an indication for reducing the dose of the drug in older people. For patients with renal failure of varying severity (creatinine clearance from 9 to 55 ml/min) there were no differences in the rate of elimination of a single dose of finasteride compared to healthy volunteers.
Plasma protein binding also did not differ between patients in these groups. This is explained by the fact that in patients with renal failure, a proportion of finasteride metabolites, normally excreted in the urine, is excreted in the feces. This is confirmed by an increase in the amount of finasteride metabolites in the feces in these patients, while their concentration in the urine decreases. In connection with the above, in patients with renal failure who are not indicated for hemodialysis, no dose adjustment of Proscar is required.
Indications
Treatment of benign prostatic hyperplasia for the purpose of:
- prevention of urological complications (reducing the risk of acute urinary retention) and reducing the need for surgical operations (including transurethral resection of the prostate gland and prostatectomy);
- reducing the size of an enlarged prostate gland, improving urine flow and reducing the severity of symptoms associated with benign prostatic hyperplasia. Therapy with Proscar is indicated for patients with an enlarged prostate gland.
Dosage regimen
The recommended dose is 5 mg (1 tablet) 1 time/day, regardless of meals. Proscar can be used as monotherapy or in combination with doxazosin.
For patients with renal failure of varying severity (with a decrease in CC to 9 ml/min), individual dose selection is not required, since pharmacokinetic studies have not revealed any changes in the distribution of finasteride in this category of patients.
There is also no need for dose adjustment in elderly patients, although pharmacokinetic studies indicate that the elimination of finasteride is slightly reduced in patients over 70 years of age.
Side effect
The PLESS study assessed safety in 1524 patients receiving Proscar 5 mg/day and 1516 patients receiving placebo for 4 years. 4.9% (74) of patients discontinued treatment due to adverse events attributable to Proscar, compared with 3.3% (50) of patients receiving placebo.
However, 3.7% (57) of patients taking Proscar and 2.1% (32) of patients taking placebo had treatment discontinued due to sexual dysfunction, which was the most commonly observed side effect. In the first year of treatment, impotence, decreased libido and impaired ejaculation were more common in patients taking Proscar compared to placebo.
When using Proscar in years 2-4 of the study, the incidence of the above side effects in patients taking Proscar did not differ from that in patients taking placebo.
- From the reproductive system: testicular pain, impotence, decreased libido, ejaculation disorders, decreased ejaculate volume.
- From the endocrine system: enlargement and tenderness of the mammary glands. Allergic reactions: rash, itching, urticaria, angioedema of the lips and face.
With long-term use of Proscar, there is no increase in the frequency and severity of side effects, and the number of cases of sexual dysfunction associated with taking the drug decreases with prolonged therapy. The safety and tolerability profile of therapy with combined treatment with finasteride at a dose of 5 mg/day and doxazosin 4 or 8 mg/day is comparable to the safety and tolerability of each of these drugs separately. In a 7-year placebo-controlled study (which included 18,882 healthy men), prostate cancer was detected in 18.4% of patients receiving Proscar and 24.4% of patients receiving placebo based on the results of biopsies (9060 men).
280 men (6.4%) in the Proscar group and 237 men (5.1%) in the placebo group had prostate cancer graded Gleason score 7–10 on biopsy. In approximately 98% of all cancers, the tumor was intracapsular (stage T1 or T2). Laboratory indicators: decreased levels of prostate-specific antigen.
Contraindications
- hypersensitivity to the components of the drug. Proscar is not indicated for use in women or children.
The drug should be prescribed with caution to patients with a large volume of residual urine and/or significantly reduced urine flow (careful monitoring for obstructive uropathy is required).
Pregnancy and lactation
Women of childbearing age and pregnant women should avoid contact with crushed or broken Proscar tablets due to the possibility of finasteride entering the pregnant woman's body and subsequent risk to the development of the male fetus. Due to the ability of 5-alpha reductase type II inhibitors to suppress the conversion of testosterone to dihydrotestosterone, these drugs, including finasteride, can cause pathology in the intrauterine formation of the external genitalia in the male fetus. Proscar tablets are coated and this prevents contact with finasteride, provided that the tablets are not crushed or lost integrity.
Use for renal impairment
For patients with renal failure of varying severity (with a decrease in CC to 9 ml/min), individual dose selection is not required, since pharmacokinetic studies have not revealed any changes in the distribution of finasteride in this category of patients.
The drug should be prescribed with caution to patients with a large volume of residual urine and/or a significantly reduced urine flow.
special instructions
Effect on the level of prostate-specific antigen and diagnosis of prostate cancer To date, no positive clinical effect of Proscar has been shown in patients with prostate cancer. In patients with BPH and elevated PSA levels in controlled clinical trials (with multiple PSA determinations and prostate biopsies), Proscar treatment did not affect the incidence of prostate cancer.
The overall incidence of prostate cancer did not differ significantly between the groups of patients receiving Proscar or placebo. Before starting treatment with Proscar and periodically during treatment, a rectal examination should be performed, as well as examination by other methods to detect the presence of prostate cancer. Serum PSA determination is also used to detect prostate cancer.
In general, if the initial PSA level is above 10 ng/ml, a more extensive examination of the patient is required, including, if necessary, a biopsy. If the PSA level is between 4-10 ng/ml, further examination of the patient is recommended. There is a significant overlap in PSA levels between patients with prostate cancer and patients without the disease. Therefore, in men with BPH, normal PSA values do not rule out prostate cancer, regardless of treatment with Proscar. A baseline PSA level below 4 ng/ml does not exclude the possibility of prostate cancer. Proscar causes a decrease in serum PSA of approximately 50% in patients with BPH, even in the presence of prostate cancer. In this regard, it should be borne in mind that a decrease in PSA levels in patients with BPH treated with Proscar does not exclude concomitant prostate cancer.
It has been shown that in patients receiving Proscar for 6 months or more, PSA values should be doubled to compare them with normal values for this indicator in patients not receiving treatment. This adjustment preserves the sensitivity and specificity of the PSA test and the ability to detect prostate cancer. Any prolonged increase in PSA levels in patients treated with finasteride requires careful evaluation to determine the cause, which may be non-compliance with Proscar.
Proscar does not significantly reduce the percentage of free PSA (the ratio of free to total PSA). This indicator remains constant even under the influence of Proscar. If the percentage of free PSA is used to diagnose prostate cancer, adjustment of the values of this indicator is not necessary.
Effect on laboratory parameters Serum PSA concentration correlates with the patient's age and prostate volume, and prostate volume, in turn, depends on the patient's age. When determining PSA levels, it should be taken into account that this indicator decreases in patients taking Proscar.
In most patients, the PSA level declines rapidly during the first months of therapy, after which it stabilizes at a new level, which is usually half the value measured before the start of therapy. Therefore, in patients taking Proscar for 6 months or more, the PSA level should be doubled to compare it with normal values in men not taking Proscar.
Overdose
Patients received Proscar in a single dose of up to 400 mg and in multiple doses of up to 80 mg/day for 3 months, with no adverse effects detected. There are no specific treatment recommendations for Proscar overdose.
Drug interactions
No clinically significant interactions of Proscar with other drugs were found. Apparently, Proscar does not have a noticeable effect on the activity of isoenzymes of the cytochrome P450 system and on the pharmacokinetics of drugs in the metabolism of which these isoenzymes take part.
With the combined use of Proscar with propranolol, digoxin, glyburide, warfarin, theophylline, ACE inhibitors, acetaminophen, acetylsalicylic acid, alpha-blockers, beta-blockers, calcium channel blockers, nitrates, diuretics, histamine H2 receptor blockers, HMG-CoA inhibitors -reductase, NSAIDs, quinolones and benzodiazepines, no clinically significant manifestations of drug interactions were found.
Storage conditions and periods
The drug should be stored in a place protected from light and out of reach of children at a temperature not exceeding 30°C. Shelf life: 3 years.