Glutoxim: properties of the drug
Glutoxim is a drug that affects the regulation of metabolic processes occurring in the tissues and cells of the body. In addition, Glutoxim is capable of: - restoring sensitivity to regulatory and transport molecules - peptides; - enhance bone marrow creation; - increase phagocytosis; — normalize the level of monocytes, macrophages and neutrophils; - stimulate the work of cytokines. In addition, this drug has a high tropism towards the cells of the central organs of the immune system.
Pharmacokinetics of the drug
After intramuscular, intravenous or subcutaneous administration, the bioavailability of the drug reaches 90 percent. That is, 10 percent of the active component is not absorbed by the human body, but is excreted. There is a linear relationship between the concentration of the drug in the blood and the concentration of the solution used.
When the drug is administered intramuscularly, its maximum concentration is reached after seven to ten minutes, and when administered intravenously, after two to five minutes.
This medication is metabolized in the organs and tissues of the body, and its breakdown products are excreted exclusively through the kidneys.
Glutoxim: indications for use
First of all, taking the drug Glutoxim is intended for the treatment and preventive measures of secondary immunodeficiency conditions, in order to restore suppressed immunity and disorders in bone marrow hematopoiesis. This drug is also used: - to restore the body’s lost abilities to resist various pathological effects of infectious agents; - as a hepatoprotective agent for hepatitis B and C of a chronic nature; — to prevent the occurrence of purulent complications after operations; — for antibacterial therapy of lung diseases of a chronic obstructive nature; — for complex treatment of tuberculosis; - to prevent possible exacerbations of hepatitis, which is characterized by a chronic nature; - for the treatment of toxicosis arising from tuberculosis; - as a remedy that is part of therapy aimed at treating psoriasis.
Glutoxim®
According to the results of fundamental and clinical studies, Glutoxim® allows one to overcome the drug resistance of Mycobacterium tuberculosis to isoniazid, associated with the katG (catalase-peroxidase gene) and inhA (enol-ACP reductase gene) genes.
Glutoxim® enhances the secretion of cationic peptides - defensins and catalecidins by macrophages, stimulates their absorption by Mycobacterium tuberculosis, determining the indirect antibacterial effect of the drug.
Glutoxim® potentiates the effect of chemotherapy drugs - isoniazid, rifampicin, rifabutin, cycloserine, capreomycin, levofloxacin, cationic antimicrobial peptide catalecidin on Mycobacterium tuberculosis.
As part of complex therapy for patients with tuberculosis, Glutoxim® reduces the time for resorption of infiltrative and focal changes in the lungs. At the end of the first two months of therapy, resorption of infiltrative and focal changes occurred in 93.2% of patients who received Glutoxim® along with chemotherapy, and only in 62.2% of patients in the control group who received only chemotherapy (p <0.05).
During the intensive chemotherapy phase, Glutoxim® increases the frequency of cessation of bacterial excretion and reduces the time of abacillation. By the end of the 2nd month of treatment, among patients in whom Glutoxim® was added to chemotherapy, bacterial excretion ceased in 91.1% of patients with drug-sensitive tuberculosis (vs. 61.1% in the control group) and in 75.1% of patients with drug-resistant tuberculosis (vs. 38.9% in control) (p<0.05). The acceleration of the cessation of bacterial excretion determines the high clinical and economic effectiveness of the drug.
Glutoxim® reduces the time it takes to close decay cavities. At the end of 4 months of polychemotherapy, closure of the decay cavities occurred in 88.3% of patients receiving Glutoxim®, and only in 70.8% of patients in the control group (p<0.05), receiving only chemotherapy.
When using Glutoxim, the tolerability of anti-tuberculosis chemotherapy improves. Toxic reactions (increased activity of ALT, AST, bilirubin, creatinine in the blood serum) were observed in 11.1% of patients receiving chemotherapy, and only in 4.3% of patients whose treatment regimen included Glutoxim®. Toxic-allergic reactions (eosinophilia, loss of appetite, heaviness and pain in the right hypochondrium, nausea caused by the use of rifampicin and high doses of isoniazid) when prescribing the drug Glutoxim® are observed significantly less frequently, in 6.4% - compared with 20% in the control group (p<0.05 ). Symptoms of intoxication (fever, weakness, poor health) are eliminated mainly in the first 2 months of polychemotherapy. Glutoxim® prevents exacerbation of chronic hepatitis during chemotherapy for tuberculosis, and in case of already developed drug-induced liver damage, it allows chemotherapy to be continued in full without resorting to its temporary cancellation.
In general, better tolerability allows for full anti-tuberculosis chemotherapy.
Glutoxim® (in combination with chemotherapy) allows patients to be prepared for surgical treatment in a short time (the drug increases the frequency of abacillation and promotes the disappearance or significant reduction in the frequency and intensity of clinical and laboratory symptoms of the disease). Glutoxim® improves the immediate results of surgical treatment, increases the rate of restoration of pneumatization of the lung tissue by the 21st day of the postoperative period, increases the rate of recovery without complications after surgical treatment (64.9% vs 41.4% in control), reduces the rate of specific postoperative complications (including including pleural empyema) in patients with drug-resistant fibrous-cavernous pulmonary tuberculosis (6.9% vs 32.0% in controls). The average length of stay in the surgical hospital for patients receiving Glutoxim® along with chemotherapy was 42.1±1.7 bed days, and for patients receiving chemotherapy without the addition of Glutoxim® - 62.3±8.1 (p < 0.05) bed days.
Glutoxim® improves the tolerability of chemotherapy, radiation and chemoradiotherapy in oncology. The drug allows you to reduce the incidence of leukopenia and thrombocytopenia or shift deep hematotoxicity to the moderate region, and also promotes a more rapid restoration of the content of leukocytes, platelets and erythrocytes in the peripheral blood. The use of Glutoxim during chemotherapy according to the etoposide + cisplatin regimen in patients with morphologically confirmed stage IIIb-IV non-small cell lung cancer, who had not previously received chemotherapy and radiation therapy, reduced the incidence of deep neutropenia (WHO toxicity grades 3 and 4) by 2.5 times. (p = 0.002).
Glutoxim® helps prevent or reduce the severity of reactions of the skin (dermatitis) and mucous membranes (mucositis, stomatitis, rectitis, cervicitis) to radiation and the effects of chemotherapy. When using the drug Glutoxim®, the incidence of bleeding gums and the occurrence of focal epitheliitis significantly decreased. Erosions, ulcers, necrosis and confluent epitheliitis did not occur in any of the patients with stage III-IV oropharyngeal cancer who received Glutoxim®. The frequency of complaints such as dry mouth, pain when swallowing, and changes in taste decreased.
By improving tolerability, Glutoxim® facilitates the delivery of chemotherapy, radiation and chemoradiotherapy in full, without reducing the planned courses.
Clinical studies have demonstrated an increase in the rate of positive responses to radical radiation therapy for locally advanced cervical cancer. The rate of achieving complete remission was 77% (in the control group - 38%). A faster recovery of peripheral blood parameters and an improvement in the general condition of patients during radiation therapy were noted.
The frequency of positive responses to radiation therapy in patients with stage III-IV oropharyngeal cancer (complete + partial remission) in the group of patients receiving Glutoxim® was significantly higher than in the control group (83.4% and 61.5%, respectively).
In patients with HER-2(-) stage II–III breast cancer, the addition of Glutoxim® to neoadjuvant therapy led to a doubling of the previously achieved rate of complete morphological remission.
When chemotherapy patients with platinum-resistant ovarian cancer included the drug Glutoxim®, the median relapse-free survival was 15.4 weeks, which is longer when compared with historical control data of 8 weeks in patients with the same intensity of treatment. The average disease-free survival period was 19.4 weeks. The inclusion of the drug Glutoxim® in the chemotherapy regimen showed clinical feasibility (complete remission + partial remission + stabilization) in 60% of patients.
According to the results of clinical studies, when Glutoxim was prescribed, patients with psoriasis experienced a more rapid and complete regression of rashes (infiltration, peeling, swelling), itching decreased, and the quality of life improved. In the arthropathic form, the intensity of arthralgia decreased and the length of stay of patients in the hospital decreased. A more rapid regression of psoriasis symptoms is also shown in children who received Glutoxim® as part of complex therapy.
A significant increase in the duration of remission of psoriasis was demonstrated: when Glutoxim was added to therapy, the period of remission in 48% of patients exceeded 1 year; with traditional therapy, in only 18% of patients the period of remission exceeded 1 year. In case of liver damage accompanying psoriasis, Glutoxim® demonstrated hepatoprotective and toxicogenic effects.
As part of complex therapy for sexually transmitted infections, Glutoxim®, by promoting exocytosis of intracellularly located parasites, increases the effectiveness of antibacterial drugs and significantly reduces the risk of recurrent urogenital infections. Glutoxim has been shown to have a positive effect on the morphological parameters of sperm and the reproductive function of patients (an increase in the total number of sperm by 20%, a decrease in the content of leukocytes in sperm by 90%, and immature sperm by 50%).
Glutoxim: instructions for use
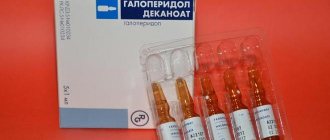
This drug is administered intravenously, subcutaneously or intramuscularly. The dosage is prescribed by a doctor (5-40 mg) and is determined depending on the specific characteristics of the body, as well as the severity and nature of the disease. The general course of the drug is 50-300 mg. In the complex treatment of tuberculosis, Glutoxim is taken daily, at a dose of 60 mg, 2 times 30 mg for 2 months. When specific inflammation enters the productive phase, this drug is administered intramuscularly. Injections are carried out 1-2 times a day, three times a week, 10-20 mg daily dose. If the need arises, this course of treatment can be repeated after a 1-6 month break. In the complex treatment of psoriasis, Glutoxim is administered intramuscularly every day at a dose of 10 mg per day for 15 days. The required course of treatment is 25 injections. For preventive purposes, this drug is administered intramuscularly every day at a dose of 5-10 mg together with water-soluble drugs.
Product concentration
The concentration of certain components in the drug may vary.
- Glutoxim solution 1% contains 10 milliliters of active substance.
- A 0.5% solution contains 5 milliliters of the active ingredient.
- Glutoxim solution 3% contains 30 milliliters of active substance.
When opening the ampoule, a slight aroma of acetic acid may emanate from the medicine. You can purchase this medicine in a cardboard box with 10 or 5 ampoules inside.
Glutoxim: cost and sale
Glutoxim, the price of which depends on the volume of the ampoule, costs 1000-2000 rubles for 5 ampoules of the drug. Buying Glutoxim today is quite simple, you just need to contact a pharmacy, a specialized department of a supermarket, or fill out an application to purchase the drug in an online pharmacy. You should not neglect information about the presence of a large number of counterfeit products on the modern pharmacological market and do not buy any medical drug, including Glutoxim, from your hands and at unverified points of sale. It is better to contact official representatives of the manufacturer, where the buyer will be presented with the necessary documents confirming the quality of the product and compliance with the shelf life of the drug. Despite the fact that the drug Glutoxim has received very favorable reviews, it still has some contraindications and side effects. Therefore, before you buy Glutoxim, the price of which is the same in different places where the product is sold, you need to consult a doctor. It is also worth studying the manufacturer's instructions, since these instructions are provided solely for the purpose of familiarizing the buyer with this drug.
Features of the drug
What is the opinion of patients about the drug Glutoxim? Their reviews are different. Some men who have been prescribed this medication for the treatment of kidney disease note that it improves erection. Experts have proven that this drug increases the number of live sperm in semen, and also reduces the severity of side effects after using antibiotics. In addition, this medication can increase the effectiveness of antibacterial drugs that belong to the quinolone series (Ofloxacin, Oxolinic acid, Norfloxacin and Cyclofloxacin).
This drug accelerates remission in the treatment of chronic kidney diseases. Its use significantly reduces the frequency of relapses, up to their complete disappearance.
According to expert reviews, the drug "Glutoxim" is very effective in the treatment of chronic prostatitis, as well as infectious diseases that are sexually transmitted.
Glutoxim and Transfer Factor
For a course of therapy, you will need to take about 20 ampoules of the drug Glutoxim, the price of which is 1000-2000 rubles for 5 ampoules. The total cost of treatment will be about 4000-8000 rubles. Glutoxim is not a cheap drug, and to achieve results, the drug may need to be prescribed again, which will certainly increase the cost of treatment.
Transfer factor, which performs the same functions, costs about 2,000 rubles per pack of 90 capsules. However, there are no restrictions on taking the drug. It can be taken by people of any age, as well as children, pregnant and lactating women. The drug has no side effects, is not addictive and an overdose of Transfer Factor is excluded. The drug affects the human body much deeper and more dramatically than any similar drugs. Transfer factor repairs damage in the DNA structure, which allows not only to restore the functioning of the immune system, but also to eliminate the causes of the disease.
Taking Glutoxim together with Transfer Factor will allow the latter to neutralize all side effects that are associated with taking Glutoxim, and will quickly restore the body's immune system.
Use of the medicine for children
Is it possible to give Glutoxim injections to children? Of course yes. But this is only if other drugs have proven ineffective.
As for the volume of use of the drug "Glutoxim", the dosage for children is calculated based on weight (2.5-5 milligrams per kilogram). In this case, the drug can be administered intramuscularly, intravenously or subcutaneously.
During the treatment of kidney disease in young children and adolescents, the use of this drug is associated with reduced effectiveness of antibiotic and hormonal treatment. Medical practice shows that the use of this medicine accelerates the onset of remission, and also improves the general condition of patients, without affecting the blood count.
Patient reviews
Patients who have ever used this drug after chemotherapy note that compared to the first course, when this drug was not yet prescribed, the condition has become more comfortable. At the same time, the taste in the mouth and dry mucous membranes completely disappeared. The patients’ sleep also noticeably improved, and the habitual state of anxiety that occurred every time after completing chemotherapy went away.
Those patients who were prescribed the drug "Glutoxim" for the treatment of prostate tumors note an impressive result. The growth of the tumor slowed down, and the inflammation subsided.
Patients diagnosed with pulmonary tuberculosis also talk about the effectiveness of the medicine. According to their reviews, treatment of the disease is quick and comfortable. In patients, the normal blood formula is restored, signs of intoxication disappear, and sleep improves and the unpleasant bitterness in the mouth and dry mucous membranes disappear.
Release form and composition
Glutoxim is produced in the form of an injection solution: a transparent, slightly colored or colorless liquid with a faint acetic odor or practically odorless (in neutral glass ampoules of 1 or 2 ml, 5 or 10 ampoules in a strip pack, 1, 2, 5 or 10 packages in a cardboard box complete with a scarifier or ampoule knife)
The composition of 1 ml of injection solution includes the active ingredient: Glutamyl-Cysteinyl-Glycine disodium - 5, 10 or 30 mg and auxiliary components: diluted acetic acid, sodium acetate, water for injection.
Additional information about the medicine
Currently, research into the effect of the mentioned drug on the human body is ongoing. The instructions that come with the medicine contain the most studied methods of taking the medicine. There is evidence of the effectiveness of its use in the treatment of infectious diseases, including those that are sexually transmitted, as well as various hepatitis, tumors and other disorders.