pharmachologic effect
Hormonal contraceptive drug.
Etonogestrel is a progestogen that binds to progesterone receptors in target organs.
Ethinyl estradiol is an estrogen. The effect of the drug is ensured by a combination of various mechanisms, the most significant of which is the suppression of ovulation.
The use of the drug regulates the menstrual cycle, reducing pain during menstrual-like bleeding and its intensity. This reduces the likelihood of developing iron deficiency anemia .
The use of the drug reduces the risk of ovarian and endometrial cancer, the development of ovarian cysts , ectopic pregnancy , inflammation of the female reproductive system and benign pathologies of the mammary glands.
How the ring works and its mechanism of action
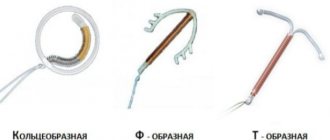
The Nuvaring contraceptive ring is a thin translucent ring with a diameter of approximately 6 cm. It is a flexible elastic ring made of a special synthetic material widely used for the production of various medical implants. The material is quite hypoallergenic, so allergies to the contraceptive ring are a very rare occurrence.
Inside the ring there is a medicinal substance - ethinyl estradiol and etonogestrel. These are hormonal substances that are released daily from the pores of the material in quantities strictly stated by the manufacturer and enter the bloodstream through the rich vascular network of the vagina.
This mechanism is similar to taking a birth control pill every day, only the medicine is absorbed into the bloodstream not through the gastrointestinal tract, but through the vagina.
The hormones in the ring have the following contraceptive effects:
- Suppresses ovulation processes in the ovaries.
- They prevent the growth of the endometrium, thereby preventing implantation of the embryo.
- They create an artificially simulated hormonal background.
- They thicken the mucus in the cervical canal and prevent sperm from penetrating into the uterine cavity and tubes.
All these effects are absolutely reversible. By stopping hormonal pills or stopping using the ring, a woman can easily become pregnant within 1-3 cycles.
Pharmacodynamics and pharmacokinetics
Etonogestrel is absorbed through the vaginal mucosa and its maximum concentration is reached after approximately 7 days. Bioavailability is about 100%, which is higher than with oral etonogestrel. The substance is metabolized in the liver and excreted in urine and bile. The half-life of metabolites is 6 days.
Ethinyl estradiol also penetrates the vaginal mucosa into the blood. The maximum concentration is reached after 3 days. Bioavailability is approximately 56% and is comparable to oral administration. Metabolized in urine and bile, the half-life is about 36 hours.
Use during pregnancy and breastfeeding
Nuvaring is designed to protect the female body from unwanted pregnancy.
If you want to get pregnant, women are advised to first wait until the natural menstrual cycle is restored, then start calculating the possible date of conception and birth.
The drug should not be used once pregnancy occurs, as soon as it is known.
In this condition, it is important to stop contraception in a timely manner, despite the absence of cases of detecting defects in newborns.
Nuvaring is administered topically, into the vagina, and is considered a safe drug, but it is not advisable to use it while breastfeeding. The active ingredients will negatively affect lactation and reduce the production of breast milk.
It is known that metabolites and steroids are excreted in breast milk, although a negative impact on the health of children has not been proven.
Contraindications
- arterial and venous thrombosis , thromboembolism and predisposition to them;
- heart defects with complications such as thrombosis;
- migraine;
- diabetes mellitus with vascular changes;
- pancreatitis;
- severe liver disease;
- malignant and benign liver tumors;
- hormone-dependent malignant tumors (breast and reproductive system);
- vaginal bleeding of unknown origin;
- pregnancy;
- hypersensitivity to any of the substances of the drug (active or auxiliary).
If any of these conditions occur, stop taking the drug immediately.
Prescribe the drug with caution in the following cases:
- a family history of venous or arterial thrombosis ;
- major surgical interventions and interventions on the lower extremities, prolonged immobilization;
- obesity with a body mass index over 30;
- thrombophlebitis;
- arterial hypertension;
- smoking (especially women over 35 years old);
- lipid metabolism disorders;
- heart defects;
- atrial flutter;
- diabetes;
- liver dysfunction;
- cholelithiasis;
- hemolytic-uremic syndrome;
- chorea;
- otosclerosis with hearing loss;
- systemic lupus erythematosus;
- angioedema;
- ulcerative colitis and Crohn's disease ;
- sickle cell anemia;
- porphyria;
- chloasma;
- cases of difficult use of the vaginal ring: severe chronic constipation , hernia of the bladder and rectum, cervical prolapse.
In case of exacerbation of diseases and deterioration of the condition, or if one of the above-mentioned conditions occurs for the first time, further questions about taking the drug must be resolved with a doctor.
NuvaRing®
If any of the following conditions/diseases or risk factors are present, a thorough assessment of the benefit-risk ratio of using the drug NuvaRing® should be carried out and discussed with the woman before starting to use the drug. In case of exacerbation of diseases, deterioration of the condition or the appearance of the first symptoms of conditions/diseases or risk factors, the woman should immediately consult a doctor to decide whether to discontinue or the possibility of further use of the drug.
Risk of developing VTE and ATE
The use of any combined hormonal contraceptives (CHCs) is associated with an increased risk of developing venous and arterial thrombosis and thromboembolism (such as DVT and PE, myocardial infarction, cerebrovascular disorders). These diseases are rare. The use of drugs containing levonorgestrel, norgestimate or norethisterone as a progestogen component has the lowest risk of developing VTE.
The use of other medications, such as NuvaRing®, can double the risk of developing this complication. An increased risk is present after initial use of CHCs or resumption of use after a break of 4 weeks or more. The choice in favor of a CHC with a higher risk of developing VTE can only be made after consultation with the woman to ensure that she fully understands the risk of VTE associated with the use of NuvaRing®; the effect of the drug on her existing risk factors and that the risk of developing VTE is greatest in the first year of using the drug.
VTE can be life-threatening or lead to death (in 1-2% of cases).
Thrombosis of other blood vessels, for example, hepatic, mesenteric, renal, cerebral veins and arteries or retinal vessels, has occurred extremely rarely with the use of CGCs.
Symptoms of deep vein thrombosis (DVT):
unilateral swelling of the lower limb or along the vein, pain or discomfort only in an upright position or when walking, local increase in temperature, redness or discoloration of the skin in the affected lower limb.
Symptoms of pulmonary embolism (PE)
: difficulty or rapid breathing; sudden cough, including with hemoptysis; sharp pain in the chest, which may intensify with deep inspiration; sense of anxiety; severe dizziness; fast or irregular heartbeat. Some of these symptoms (eg, shortness of breath, cough) are nonspecific and may be misinterpreted as signs of other more common and less severe conditions (eg, respiratory tract infection).
ATE can lead to stroke, vascular occlusion, or myocardial infarction.
Symptoms of a stroke:
sudden weakness or loss of sensation in the face, limbs, especially on one side of the body, sudden confusion, severe or prolonged headache for no apparent reason, unilateral or bilateral loss of vision; problems with speech and understanding; sudden disturbance in gait, dizziness, loss of balance or coordination; sudden loss of consciousness or fainting with or without a seizure.
Other signs of vascular occlusion
: sudden pain, swelling and slight cyanosis of the extremities, “acute” abdomen.
Symptoms of myocardial infarction:
pain, discomfort, pressure, heaviness, a feeling of compression or fullness in the chest or behind the sternum, radiating to the back, jaw, upper limb, epigastric region; cold sweat, nausea, vomiting or dizziness, severe weakness, anxiety or shortness of breath; fast or irregular heartbeat.
ATE can be life-threatening and lead to death.
In women with a combination of several risk factors or high severity of one of them, the possibility of their mutual reinforcement should be considered. In such cases, the degree of increase in risk may be higher than with a simple summation of factors. In this case, the use of NovaRing® is contraindicated.
The risk of developing thrombosis (venous and/or arterial) and thromboembolism or cerebrovascular disorders increases:
- with age;
- in women who smoke (with an increase in the number of cigarettes or an increase in age, the risk increases, especially over the age of 35);
- if there is a family history (for example, venous or arterial thromboembolism in close relatives or parents under the age of 50 years). In the case of a hereditary or acquired predisposition, the woman should be examined by an appropriate specialist to decide on the possibility of using CHC;
— for obesity (UTI more than 30 kg/m2);
- with dislipoproteinemia;
- for arterial hypertension;
- for migraine;
- for diseases of the heart valves;
- with atrial fibrillation;
- in case of prolonged immobilization, major surgery, any operation on the lower extremities or major trauma. In these cases, the use of CHCs should be discontinued (in the case of planned surgery, at least four weeks before it) and not resumed for two weeks after the end of immobilization. Temporary immobilization (eg, air travel lasting more than 4 hours) may also be a risk factor for the development of VTE, especially in the presence of other risk factors.
The possible role of varicose veins and superficial thrombophlebitis in the development of VTE remains controversial.
The increased risk of thromboembolism in the postpartum period should be taken into account.
Peripheral circulatory disorders may also occur in diabetes mellitus, systemic lupus erythematosus, hemolytic uremic syndrome, chronic inflammatory bowel disease (Crohn's disease or ulcerative colitis) and sickle cell anemia.
An increase in the frequency or severity of migraine (which may also precede cerebrovascular events) during the use of CHCs is grounds for immediate discontinuation of these drugs.
Biochemical indicators indicating a hereditary or acquired predisposition to the development of venous or arterial thrombosis include: resistance to activated protein C, hyperhomocysteinemia, antithrombin III deficiency, protein C deficiency, protein S deficiency, antiphospholipid antibodies (anticardiolipin antibodies, lupus anticoagulant).
The risk of thrombosis and thromboembolism during pregnancy and the postpartum period is higher than when taking low-dose CHCs (containing less than 0.05 mg ethinyl estradiol).
If VTE or ATE is suspected or confirmed, use of the drug should be stopped immediately. In this case, it is necessary to use effective contraception, since anticoagulants (coumarins) have a teratogenic effect.
Risk of developing tumors
The most important risk factor for developing cervical cancer is infection with the human papillomavirus (HPV). Epidemiological studies have shown that long-term use of COCs leads to an additional increase in this risk, but it is unclear how much of this is due to other factors, such as increased frequency of cervical screening and sexual behavior, including the number of sexual partners and less frequent use of barrier contraceptives. and their cause-and-effect relationship. Epidemiological data regarding the incidence of cervical cancer in women using the drug NuvaRing®
There are no data from epidemiological studies regarding the risk of developing cervical cancer in women using the drug NuvaRing®.
A meta-analysis of the results of 54 epidemiological studies found a small increase (1.24) in the relative risk of developing breast cancer in women taking COCs. The risk gradually decreases over 10 years after stopping the drugs. Breast cancer rarely develops in women under 40 years of age, so the additional incidence of breast cancer in women who take or have taken COCs is small compared to the overall risk of developing breast cancer. Women who use COCs are diagnosed with earlier clinical stages of breast cancer than women who have never used COCs. The observed increased risk may be due to earlier diagnosis of breast cancer in women taking COCs, the biological effects of COCs, or a combination of both.
In rare cases, cases of development of benign, and even more rarely, malignant liver tumors have been observed in women taking COCs. In some cases, these tumors led to the development of life-threatening bleeding into the abdominal cavity. The doctor should consider the possibility of a liver tumor in the differential diagnosis of diseases in a woman taking NuvaRing® if symptoms include acute pain in the upper abdomen, liver enlargement, or signs of intra-abdominal bleeding.
Hypersensitivity reactions
Hypersensitivity reactions, such as angioedema and anaphylaxis, have been observed during use of the drug NovaRing®. If you suspect angioedema and/or anaphylaxis, you should stop using the drug NuvaRing® and carry out appropriate treatment.
Hepatitis C
In clinical trials of the drug combination for the treatment of hepatitis C virus ombitasvir/paritaprevir/ritonavir with or without dasabuvir, increases in ALT levels greater than 5 times the upper limit of normal were observed significantly more often in women using drugs containing ethinyl estradiol, such as COOK. The use of NovaRing® should be discontinued before starting therapy with the combination of ombitasvir/paritaprevir/ritonavir with or without dasabuvir (see section “Contraindications” and “Interaction with other drugs”). The use of NuvaRing® can be resumed approximately 2 weeks after completion of treatment with the combination of these drugs.
Other states
- In women with hypertriglyceridemia (or a family history of this condition), the risk of developing pancreatitis may increase while using CHCs.
- Although slight increases in blood pressure (BP) have been described in many women using COCs, clinically significant increases in BP have been reported rarely. However, if a persistent clinically significant increase in blood pressure develops while using the drug NuvaRing®, the use of the drug should be stopped and treatment of arterial hypertension should be started. If normal blood pressure values are achieved with the help of antihypertensive therapy, use of the drug NuvaRing® can be continued.
- The following conditions have been reported to develop or worsen during pregnancy and when taking CHCs, but their relationship with the use of CHCs has not been proven: cholestatic jaundice and/or pruritus associated with cholestasis; formation of gallstones; porphyria; systemic lupus erythematosus; hemolytic-uremic syndrome; chorea; herpes during pregnancy; hearing loss associated with otosclerosis. Cases of Crohn's disease and ulcerative colitis have also been described with the use of CHCs.
— In women with hereditary forms of angioedema, exogenous estrogens may cause or worsen symptoms of angioedema.
— Acute or chronic liver diseases may require discontinuation of the drug NuvaRing® until liver function tests return to normal.
— Recurrence of cholestatic jaundice and/or itching caused by cholestasis, which first developed during pregnancy or previous use of sex hormones, requires discontinuation of the drug NuvaRing®.
- Although estrogens and progestogens can affect insulin resistance and glucose tolerance, in patients with diabetes mellitus using low-dose CHCs, as a rule, no dose adjustment of hypoglycemic drugs is required. However, patients with diabetes mellitus should be carefully monitored while using NuvaRing®.
- Chloasma can sometimes develop, especially in women with a history of chloasma during pregnancy. Women with a tendency to chloasma should avoid prolonged exposure to the sun and ultraviolet irradiation while using NuvaRing®.
— Conditions of a woman in which she will not be able to insert the ring correctly or in which the ring may fall out: cervical prolapse, bladder hernia and/or rectal hernia, severe chronic constipation.
— In very rare cases, women have unintentionally inserted the NuvaRing® vaginal ring into the urethra and possibly into the bladder. When symptoms of cystitis appear, it is necessary to consider the possibility of incorrect insertion of the ring.
— Cases of vaginitis have been described during the use of the drug NuvaRing®. There are no clinical data regarding the effect of vaginitis therapy on the effectiveness of the use of the drug NovaRing®, as well as the effect of the use of the drug NovaRing® on the effectiveness of therapy for vaginitis.
— Very rare cases of a ring sticking to the vaginal mucosa have been described, requiring its removal by a medical professional. In some cases where tissue had grown around the ring, removal was accomplished by cutting the ring without cutting the vaginal tissue.
Medical examination/consultation
Before prescribing the drug NuvaRing® or resuming its use, you should carefully review the woman’s medical history (including family history) and conduct a gynecological examination to exclude pregnancy. It is necessary to measure blood pressure, conduct an examination of the mammary glands, pelvic organs, including a cytological examination of cervical smears and some laboratory tests, to exclude contraindications and reduce the risk of possible side effects of the drug. The frequency and nature of medical examinations depend on the individual characteristics of each patient. but medical examinations are carried out at least once every 6 months. A woman should read the instructions for use and follow all recommendations. The woman should be informed that NuvaRing® does not protect against HIV infection (AIDS) and other sexually transmitted diseases.
Reduced efficiency
The effectiveness of the drug NovaRing® may decrease if the regimen is not followed (see the subsection “Deviations from the recommended regimen” of the section “Dosage and Administration”) or concomitant therapy is carried out that reduces the concentration of etonogestrel in the blood plasma (see section “Interaction with other drugs” ).
Changes in the nature of menstruation
During use of the drug NovaRing®, acyclic bleeding may occur (“spotting” spotting or sudden bleeding). If such bleeding is observed after regular cycles with the correct use of the drug NuvaRing®, you should contact your gynecologist to conduct the necessary diagnostic studies, including to exclude organic pathology or pregnancy. A diagnostic curettage may be required.
Some women do not bleed after the ring is removed. If the drug NuvaRing® was used according to the instructions, it is unlikely that the woman is pregnant. If the recommendations of the instructions are not followed and there is no bleeding after removing the ring, as well as if there is no bleeding for two cycles in a row, pregnancy must be excluded.
Effects of ethinyl estradiol and etonogestrel on a sexual partner
The possible pharmacological effects and extent of exposure of ethinyl estradiol and etonogestrel to male sexual partners (due to absorption through penile tissue) have not been studied.
Ring damage
In rare cases, when using the drug NuvaRing®, ring rupture was observed. The core of the drug NuvaRing® is solid, so its contents remain intact, and the release of hormones does not change significantly. Vaginal injury associated with ring rupture has been reported. If the ring ruptures, it usually falls out of the vagina (see the recommendations in the subsection “What to do if the ring has been temporarily removed from the vagina” in the “Dosage and Administration” section). If the ring ruptures, a new ring must be inserted.
Ring falling out
Sometimes the NuvaRing® vaginal ring may fall out of the vagina, for example, if it is inserted incorrectly, when a tampon is removed, during sexual intercourse, or due to severe or chronic constipation. In this regard, it is advisable for a woman to regularly check the presence of the NuvaRing® vaginal ring in the vagina (for example, before and after sexual intercourse). If the NuvaRing® vaginal ring falls out of the vagina, you must follow the recommendations of the subsection “What to do if the ring has been temporarily removed from the vagina” in the “Method of administration and dosage” section.
Side effects
When used, the following side effects of Nuvaring may occur, occurring with varying frequencies.
- Infections: vaginal and urinary tract infections, cervicitis .
- Immune system: hypersensitivity.
- Metabolism: increased appetite and weight gain.
- Mental disorders: decreased sex drive, mood changes, depression .
- Nervous system: headache, dizziness , migraine .
- Visual organs: visual impairment.
- Cardiovascular system: increased blood pressure, thromboembolism , feeling of “hot flashes”.
- Digestive system: nausea, bloating, abdominal pain, diarrhea, constipation, vomiting.
- Skin: acne , itchy skin , rash .
- Musculoskeletal system: pain in the limbs and back, muscle spasms.
- Urinary system: dysuria , frequent urination.
- On the part of the reproductive system and mammary glands, side effects of the ring can manifest themselves as: engorgement and soreness of the mammary glands , genital itching, dysmenorrhea , vaginal discharge, amenorrhea , spotting during sexual intercourse, heavy menstruation, uterine bleeding, premenstrual syndrome, burning sensation in the vagina, painful sensations and discomfort in the vagina.
- General condition of the body: fatigue, swelling.
There may also be discomfort and discomfort in the vagina.
Reviews from doctors
Zhilina Margarita Viktorovna, gynecologist:
“I consider this method of intrauterine contraception to be the safest. The contraceptive Nuvaring effectively has a contraceptive effect and is not capable of damaging the integrity of the vaginal tissues.”
Bogdanova Tatyana Igorevna, gynecologist:
“For a woman, the Nuvaring vaginal ring does not cause discomfort unless adverse reactions occur. But not every man can tolerate the sensation of a foreign object.”
Instructions for use Nuvaring rings (Method and dosage)
The ring is inserted once every 4 weeks into the vagina, where it remains for 21 days and then is removed. A new ring is introduced after a break of 7 days.
On the 2-3rd day after removal of the ring, bleeding begins, which is associated with the cessation of the effect of the drug.
If hormonal contraceptives , the Nuvaring contraceptive ring is administered on the first day of menstruation. It is possible to install it up to the 5th day of the cycle, but in this case it is recommended to use a condom for the first week of using the drug.
When switching from combined oral contraceptives, the ring is administered on the last day of the interval between taking hormonal contraceptives. If you take a combined hormonal contraceptive correctly and regularly and are confident that you are not pregnant, you can insert a vaginal ring on any day of the cycle.
When switching from a mini-pill , you can start using the ring on any day (on the day the IUD is removed or on the day of the next injection). In such cases, it is necessary to use barrier contraceptives for 1 week.
The instructions for Nuvaring allow the use of the ring immediately after an abortion performed in the first trimester of pregnancy.
After an abortion in the second trimester or childbirth, use can begin in the 4th week after the abortion or childbirth.
The contraceptive effect may be impaired if the regimen and rules of use are not followed.
If there was sexual intercourse during a break in using the ring, pregnancy must be ruled out.
When the ring ruptures, the release of hormones does not change. In this case, the ring usually falls out of the vagina. It needs to be replaced with a new one.
Rules for using Nuvaring
The ring can be inserted into the vagina yourself. For insertion, you need to choose a comfortable position - standing, with one leg raised, squatting or lying down. In this case, the ring must be squeezed and inserted into the vagina.
To remove the ring, squeeze it with your index and middle fingers and remove it from the vagina.
Safe days calendar
1st day of last menstruation
Average cycle length
Duration of menstruation
| 01 | Days of menstruation |
| 02 | Conditionally safe days |
| 03 | Ovulation |
| 04 | Favorable day for conceiving a girl |
| 05 | Favorable day to conceive a boy |
| 06 | Safe days to conceive |
Interaction
An interaction may occur, manifested in the onset of acyclic bleeding, with the following drugs: barbiturates , carbamazepine , phenytoin , rifampicin , oxcarbazepine , topiramate , felbamate , St. John's wort preparations. During treatment with these drugs and for 1 month after stopping therapy, a condom should be used.
When treating with antibiotics ampicillin and tetracyclines, you should use a condom during treatment and for 1 week after stopping the antibiotic, since the effectiveness of contraceptives that contain ethinyl estradiol .
The use of suppositories with antifungal drugs slightly increases the risk of ring damage and rupture.
The use of hormonal contraceptives may cause changes in the metabolism of other drugs, and their concentration in plasma or peripheral tissues may increase ( cyclosporine ) or decrease ( lamotrigine ).
Description
Most women prefer to use contraceptives, but at the same time, taking pills requires them to constantly remember that a missed dose can lead to an unwanted pregnancy.
This is why Nuvaring is currently very popular. If you decide to have a baby, then after Nuvaring you can easily get pregnant, usually in the 2nd month after you no longer use the ring. Nuvaring is a contraceptive hormonal ring that is inserted into a woman’s internal genital organs. Administration is so easy that you do not need to see a doctor for it. Just read the instructions and you will be able to do it yourself every month easily. This way, you no longer have to worry about forgetting to take your next pill. After reading Nuvaring reviews, you will instantly understand that this is exactly the drug that you have been looking for for so long.
Nuvaring is also very popular among women because it does not promote weight gain, unlike most oral hormonal contraceptives.
special instructions
Taking hormonal contraceptives may be associated with the development of venous and arterial thrombosis .
Possible symptoms of thrombosis are:
- pain and swelling in one leg;
- sudden intense chest pain;
- shortness of breath or paroxysmal cough
- dizziness , intense headaches;
- sudden loss of vision or double vision;
- speech disorders;
- sudden weakness or numbness on one side or part of the body;
- movement disorders.
If there are signs of thrombosis, stop taking hormonal contraceptives immediately and consult a doctor.
The reason to stop taking hormonal contraceptives should be an increase in the severity of migraine and the frequency of attacks.
Women with hypertriglyceridemia have an increased risk of developing pancreatitis.
If you are predisposed to developing chloasma, you should avoid exposure to ultraviolet radiation and the sun while using the ring.
The use of the drug may cause acyclic bleeding in the form of spotting or bleeding that occurs suddenly.
Analogs
Matches by level 4 ATX code:
Patentex Oval N
Nonoxynol
Erotex
Septefril
Benatex
Pharmatex
There are analogues for the pharmacological group, which includes combined oral contraceptives : Zhanin , Logest , Midiana , Novinet , Yarina .
What is better Jess, Mirena, Yarina or Nuvaring?
Jess, Mirena, Yarina and Novaring
Jess and Yarina are combined oral contraceptives. Mirena is an intrauterine device.
All contraceptives are used for the same purpose, but they are suitable for each woman differently and the comfort/discomfort from them is also different.
Have you used the contraceptive Nuvaring and was it effective?
Reviews of the Nuvaring ring
Many women turn to the medical forum with questions about using the Nuvaring ring. Reviews from doctors about Nuvaring are mostly considered to be a reliable and effective remedy with a minimum number of side effects. None of the reviews mentioned the occurrence of unwanted pregnancy while taking the drug. The hormonal ring is widely used by gynecologists for endometriosis as a factor that inhibits the development of the disease. Some women report side effects such as vaginal discomfort, weight gain and headaches.
Indications for use
The drug is an excellent and safe contraceptive and is considered the best contraceptive for women of reproductive age.
It has a low-dose combined hormonal effect, promotes:
- preventing the release of an egg from the ovary;
- protection against unwanted pregnancy;
- normalization of menstruation;
- reduction of painlessness, acyclic manifestations.
Nuvaring can be used for uterine fibroids if, of course, according to ultrasound, the tumor has stopped growing.
Although you should first consult your doctor about the advisability of using contraception. Also, the course of the disease improves with endometriosis as the hormonal ring is used.
With the arrival of menstruation, patients begin to lose significantly less blood, pain decreases and the endometrial uterine layer is restored.
The action of Nuvaring is gentle and gentle, therefore it does not lead to the failure of systems and organs, and the active components, when they come into contact with the mucous layer of the uterus, begin to have a local therapeutic effect.
Nuvaring price, where to buy
The price of the Nuvaring hormonal ring is about 1000 rubles. for 1 piece Buy a pack of 3 pieces in Moscow. possible for 2800 rubles. In Ukraine, the average cost of one ring is 220 UAH.
The average price in pharmacies in Minsk is 182,200 BYN. rub.
- Online pharmacies in RussiaRussia
- Online pharmacies in UkraineUkraine
ZdravCity
- Nuvaring vaginal ring 15mcg+120mcg/day 3 pcs.Organon
RUB 3,706 order - Nuvaring vaginal ringOrganon
RUB 1,461 order
Pharmacy Dialogue
- NuvaRing vaginal ring. (No. 1)Organon
RUB 1,352 order
- NuvaRing vaginal ring. (No. 3)Organon
RUR 3,424 order
show more
Composition and release form
The contraceptive Nuvaring is made in the form of a vaginal ring.
Nuvaring vaginal ring
The concentration of ethinyl estradiol in it is 2.7 mg, and etonogestrel is 11.7 mg.
The composition also includes additional substances in the form of:
- Ethylene copolymer.
- Vinyl acetate copolymer.
- Magnesium stearate.