The causative agent of leishmaniasis is parasitic protozoa of the genus Leishmania, which has more than 20 species. It has been established that more than 90 species of mosquitoes can carry Leishmania parasites. There are 3 main forms of the disease:
- Visceral leishmaniasis (VL), also known as kala-azar, is fatal in 95% of cases if left untreated. It is characterized by irregular bouts of fever, weight loss, enlarged spleen and liver, and anemia. Most cases occur in Brazil, East Africa and India. It is estimated that between 50,000 and 90,000 new cases of VL occur annually worldwide, but only 25–45% of these are notified to WHO. This form of leishmaniasis remains one of the parasitic infections with the highest epidemic potential and mortality. In 2018, more than 95% of new cases notified to WHO were reported in 10 countries: Brazil, China, Ethiopia, India, Iraq, Kenya, Nepal, Somalia, South Sudan and Sudan.
- Cutaneous leishmaniasis (CL) is the most common form of leishmaniasis and is accompanied by skin lesions, mainly ulcers, on exposed areas of the body. Skin lesions can leave permanent scars and lead to disability or stigmatization. About 95% of cases of CL are observed in the countries of the Americas, the Mediterranean basin, the Middle East and Central Asia. In 2021, more than 85% of new cases of CL were reported in 10 countries: Afghanistan, Algeria, Bolivia, Brazil, Colombia, Iran (Islamic Republic of), Iraq, Pakistan, and the Syrian Arab Republic and Tunisia. There are an estimated 600,000 to 1 million new cases of the disease each year worldwide.
- Mucocutaneous leishmaniasis leads to partial or complete destruction of the mucous membranes of the nose, mouth and larynx. More than 90% of cases of mucocutaneous leishmaniasis occur in Bolivia (Plurinational State of), Brazil, Ethiopia and Peru.
Transmission mechanism
Leishmania parasites are transmitted by the bites of infected female mosquitoes that feed on blood to lay eggs. The epidemiological characteristics of leishmaniasis may vary depending on the species of parasites and mosquitoes, the ecology of the areas where transmission occurs, the population's current or past exposure to the pathogen, and behavioral factors. It has been established that the natural reservoirs of Leishmania parasites are about 70 animal species, including humans.
Routes of infection
Leishmania is transmitted by mosquitoes, which become infected when they bite a sick animal or person. That is, if a mosquito that has bitten an infected person bites a healthy person, infection will occur.
Carriers of protozoan microorganisms (Leishmania) are called reservoirs. The reservoir can be any vertebrate, for example, animals - canids (foxes, jackals, dogs), rodents (gerbils, gophers).
Moreover, in reservoirs, Leishmania is in an immature flagellated form, then in the mosquito’s throat it turns into an active, motile form. When a mosquito bites a person, mature Leishmania protozoa enter the wound, enter the body and parasitize the cells, affecting the skin or internal organs.
Infected mosquitoes remain infectious throughout their lives and can transmit the disease to large numbers of people and animals.
Features of the epidemiology of the disease in different WHO regions
African region
Visceral, cutaneous and mucocutaneous forms of leishmaniasis are highly endemic in Algeria and East African countries. In East Africa, outbreaks of visceral leishmaniasis are common.
Americas region
The epidemiology of cutaneous leishmaniasis in the Americas is highly complex and highly heterogeneous with regard to transmission cycles, reservoirs, mosquito vector species, clinical manifestations and treatment response; in addition, different Leishmania species may circulate in the same geographic area. In 2021, more than 97% of VL cases in this region were reported in Brazil.
Eastern Mediterranean Region
This region accounts for 70% of all cases of cutaneous leishmaniasis in the world. Visceral leishmaniasis is highly endemic in Iraq, Somalia and Sudan.
European region
This region is endemic for cutaneous and visceral leishmaniasis. In 2018, more than 200 cases were reported in the region, mainly imported from Africa and the Americas region.
Southeast Asia region
The most common form of the disease in this region is visceral leishmaniasis, but the region is also endemic for cutaneous leishmaniasis. It is the only region with a regional initiative to eliminate visceral leishmaniasis as a public health problem by 2021. The region reported fewer than 5,000 cases in 2021, an all-time low. The region is confidently moving towards achieving its goal, and countries in the region intend to receive confirmation from the WHO of eliminating the disease by 2023.
Protection against mosquito bites
Protection from the bites of insects that carry leishmaniasis is one of the most reliable and simple measures to prevent the disease. For these purposes use:
- repellents;
- mechanical means of protection (mosquito nets).
Repellents are substances that repel insects. They are available in the form of sprays, creams, lotions. Mosquito repellents include diethyltoluamide (DEET), permethrin. Repellents are used to treat skin and clothing, and aerosols are sprayed in the house at night.
Mosquito nets are placed on windows and doors of houses, and installed in the bedside area. To increase the effectiveness of the mechanical protective agent, it is treated with an insecticide.
If a person travels to a country that is at high risk of a leishmaniasis epidemic, he must stock up on mosquito repellents. This is a minimum preventive measure.
Post-kala azar cutaneous leishmaniasis (PCCL)
Post-kala azar cutaneous leishmaniasis (PCCL) is usually a consequence of visceral leishmaniasis and manifests as a macular, papular or nodular rash, most often on the face, shoulders, trunk and other parts of the body. This clinical form of the disease is characteristic mainly of East Africa and the Indian subcontinent, where it is reported in 5-10% of patients with visceral (kala-azar) leishmaniasis. Typically, skin rashes appear between 6 months and one or more years after signs of visceral leishmaniasis have disappeared, but it can occur earlier. People with PCCL are considered a potential source of infection.
Incidence (per 100,000 people)
| Men | Women | |||||||||||||
| Age, years | 0-1 | 1-3 | 3-14 | 14-25 | 25-40 | 40-60 | 60 + | 0-1 | 1-3 | 3-14 | 14-25 | 25-40 | 40-60 | 60 + |
| Number of sick people | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 |
Main risk factors
Socio-economic conditions
Poverty is a risk factor for leishmaniasis. Poor housing conditions and unsanitary conditions (eg, lack of waste disposal systems, open sewers) can increase the number of breeding sites for mosquitoes, as well as their proximity to humans. Mosquitoes are attracted to crowded living conditions that are favorable for feeding on human blood. Behavioral factors such as sleeping outdoors or on the ground may also be associated with an increased risk of infection.
Poor nutrition
Protein-energy malnutrition and dietary deficiencies of iron, vitamin A and zinc increase the risk of developing clinical disease in the event of infection.
Population movement
Epidemics of the two most common forms of leishmaniasis are often associated with migration and the movement of non-immune people into areas where the infection is circulating. Professional activities and large-scale deforestation remain important morbidity factors.
Environmental changes
Urbanization and increased intensity of economic activity in forest areas may be factors for the incidence of leishmaniasis.
Changing of the climate
The epidemiology of leishmaniasis depends on a number of climatic factors:
- changes in temperature, precipitation and humidity can have a significant impact on the distribution area, survival and population sizes of vectors and reservoirs of infection;
- small fluctuations in temperature can have a profound effect on the developmental cycle of Leishmania promastigotes in mosquitoes, which may create conditions for transmission of the protozoa in areas not previously endemic for the disease;
- drought, famine and floods can trigger mass displacement and migration of populations into areas where leishmaniasis circulates, and poor nutrition can have a negative impact on immunity.
Dehumidification of premises
Favorable conditions for the life and reproduction of mosquitoes are created in conditions of high humidity. To live closer to the food source, humans, insects inhabit basements. To prevent epidemics of leishmaniasis, mosquito habitats are drained. Domestic or industrial dehumidifiers are used. Before drying, the room is treated, if necessary, with paradichlorobenzene, DDT emulsion or hexachlorane. These are powerful insecticides that can kill large populations of mosquitoes.
It is also important to destroy rodent habitats and seal cracks in the floors and walls.
Diagnosis and treatment
Diagnosis of visceral leishmaniasis is made on the basis of the clinical picture in combination with parasitological or serological studies (for example, rapid testing). For the diagnosis of cutaneous and mucocutaneous leishmaniasis, serological tests are not of great interest; in these cases, the diagnosis is made on the basis of the clinical picture and the results of parasitological examination.
The choice of treatment for leishmaniasis depends on a number of factors, such as the clinical form, the presence of associated pathologies, the type of parasite and the geographical area. Leishmaniasis is treatable and can be cured completely, however, the effectiveness of drugs depends on the state of the patient’s immune system, and relapses are possible if the immune system is weakened. All patients with visceral leishmaniasis are advised to immediately receive a full course of treatment. Detailed information on the treatment of different forms of leishmaniasis depending on the geographical area is given in the WHO technical report series No. 949 on the control of leishmaniasis.
Prevention and control
Prevention and control of leishmaniasis requires a multimodal approach because transmission occurs within a complex biological system involving the human or animal reservoir (hosts), the parasite and its vector (the mosquito). The main measures to prevent leishmaniasis include:
- Early diagnosis and prompt initiation of effective treatment help reduce the prevalence of the disease and prevent disability and death in patients. This provides an opportunity to reduce transmission and monitor the spread and burden of disease. There are now highly effective and safe drugs for the treatment of leishmaniasis, especially its visceral form, although their use can be difficult. WHO's efforts to harmonize prices and the WHO-brokered free drug program have greatly increased access to medicines.
- Vector control helps reduce disease or interrupt transmission by reducing mosquito populations. Insecticide spraying, insecticide-treated netting, environmental engineering measures and personal protective equipment are used to control vectors.
- Effective surveillance is important because it allows for rapid monitoring and intervention during epidemics and in situations where there are high case fatality rates among patients under treatment.
- Control of animal populations as reservoirs of infection requires a complex set of measures and therefore must be carried out taking into account local conditions.
- Social mobilization and strengthening partnerships: Community mobilization and health education and effective behavior change interventions must always be locally tailored. Working in partnership and collaboration with various stakeholders and programs to control other vector-borne diseases is critical.
WHO activities
WHO's work on leishmaniasis control is carried out in the following areas:
- technical and financial assistance to national leishmaniasis control programs to update guidance documents and develop disease control plans that include sustainable and effective surveillance systems and epidemic preparedness and response systems;
- monitoring epidemiological indicators and assessing the effectiveness of disease control activities, which contributes to increasing awareness and advocacy on the global burden of leishmaniasis, as well as ensuring equitable access to health care;
- developing evidence-based strategies and standards for the prevention and control of leishmaniasis, and monitoring their implementation;
- strengthening cooperation and coordination among partners and stakeholders;
- promoting research and use of effective treatments for leishmaniasis, including safe, effective and affordable drugs, diagnostics and vaccines;
- providing support to national disease control programs to ensure patient access to quality-assured medicines.
Cutaneous leishmaniasis ( leishmaniosis cutanea
, synonym: Pendensky ulcer, Borovsky disease) is a transmissible protozoal disease caused by Leishmania (
Leishmania tropica
), endemic to areas of tropical and subtropical climates, developing after human bites by mosquitoes infected with Leishmania, characterized by skin lesions with the formation of tubercles, nodes, and their ulceration and scarring.
Leishmaniasis is endemic in 98 countries worldwide, with an estimated 350 million people at risk of infection. According to WHO estimates, 14 million people are currently infected worldwide, and about 2 million new cases occur annually [1].
Leishmaniasis is prevalent mainly in tropical and subtropical countries. The most active foci of leishmaniasis are located in Northern and Central Africa (Morocco, Algeria, Tunisia, Libya, Egypt, Ethiopia, Sudan, etc.) and in Asia (Syria, Iraq, Israel, Jordan, Turkey, Iran, Saudi Arabia, Pakistan, India and Afghanistan) [2].
In Europe, small numbers of patients are reported in Greece, Italy, Spain, Portugal and the south of France. A special form of leishmaniasis, similar in clinical picture to cutaneous leishmaniasis of the Old World, is found in many countries of Latin America. Zoonotic cutaneous leishmaniasis is common in Turkmenistan and Uzbekistan [2]. Anthroponotic cutaneous leishmaniasis has been reported very rarely in these regions over the past 10 years [2]. It is generally accepted that in Russia, leishmaniasis (with the exception of isolated, so-called imported cases) practically does not occur1.
Historical excursion
Leishmaniasis has been known since ancient times. The first descriptions of external manifestations similar to cutaneous leishmaniasis were found on clay tablets of Ashurbanipal, dating back to the 7th century BC. There are also earlier texts dating back to 1500-2500 BC.
Cutaneous leishmaniasis is depicted in ceramic figurines of the Peruvian Incas (2000 BC), and is mentioned under various names in the written records of many peoples, for example, 1500 BC. in “Papyrus Ebers” (G. Ebers, 1874), in the Pentateuch (the execution of the god Yahweh on the heads of the Egyptians who opposed the exodus of the Jews from Egypt), “the hydra of the island of Lemne” in the “Iliad” Homeros (about 460-377 BC. ), “azar” by Alexander the Great in “Shah-nama” by Ferdowsi (994). The ancient Jews practiced inoculating “ulcers” on the skin of girls’ legs to avoid damage to the face. Cutaneous leishmaniasis was first described by the English physician Pococke (1745) during a trip to Asia Minor as “bouton d'Aleppe”. The clinical picture of the disease (“eastern ulcer”) is discussed in detail in the works of the Russel brothers (1756), Russian military doctors - in the dissertation “on salt” by N.A. Arendt in Tehran (1862, monograph “Pendin Ulcer” by L.L. Heidenreich (1888) and many other researchers [3].
Persian doctors, including Avicenna, in the 10th century AD. gave detailed descriptions of the so-called Balkh disease. Indian doctors called this disease kala-azar (kala azar in Urdu, Hindi and Hindustani means “black fever”; kala - “black”, azar - “disease, fever”). In the Americas, the earliest evidence of cutaneous leishmaniasis, dating back to the 1st century AD, is found in Ecuador and Peru in the form of images of disfigured faces and other skin manifestations on pottery of the time. There are records of the 15th-16th centuries, created during the Inca civilization and Spanish colonization, which mention “valley disease”, “Andean disease”, “white leprosy”, similar to the picture of cutaneous leishmaniasis.
The acquaintance of Russian doctors with this pathology began during the conquest of the Caucasus, as well as during the establishment of diplomatic relations between Russia and Iran. The first domestic work on cutaneous leishmaniasis belongs to Arendt, a doctor at the Russian mission in Tehran (1862). In 1864, Skorov’s article about the so-called “Elizabeth-Polish yearbook” was published. Russian doctors were aware of a peculiar skin disease found in Central Asia; however, their actual acquaintance with this disease occurred after the capture of Tashkent by General Chernyaev (1865) and the occupation of the Kokand Khanate [4].
Currently, there is no consensus on who discovered the microorganism that causes cutaneous leishmaniasis.
In 1900, the English doctor W. Leishman in London, in a preparation of the spleen of a soldier who died from “dum-dum fever” 7 months after returning from India (from Dum Dum in the vicinity of Calcutta, where English “dum-dum” bullets were produced), discovered trypanosomal parasites. In 1903, C. Donovan in Madras (India) discovered the same parasites in the spleen, and in 1905 - in the blood of patients with abnormal fever, splenomegaly and anemia ( Piroplasma
Donovani
, according to A. Laveran and F. Mensil, 1903 ) [3].
Perhaps it was first identified by British Indian Army surgeon Major Kenningham, who did not associate it with this disease. However, it is known for certain that back in the 90s. The 19th century Russian military surgeon Pyotr Fokievich Borovsky, who worked in Tashkent, conducted research on the etiology of a local disease known as “Sartov disease.” In 1898, he published the first detailed description of the parasite and the tissues it affects and classified it as a protozoan. But since the work was published in Russian and was published in a small edition, Borovsky did not receive international recognition of his works during his lifetime. Only Ronald Ross suggested that these bodies are nothing more than the intracellular stage of development of an unknown early parasite, which he called Leishmania donovani
.
Its connection with kala-azar disease was first suggested by Charles Donovan himself, but the presence of Leishmania donovani
in patients with black fever was finally confirmed by Charles Bentley.
Already by the beginning of the second half of the 19th century, the idea arose about the parasitic nature of this disease. The first reports of the parasitic nature of ulcers were of little evidence. As a curiosity, one can point to the works of Smith (Smith, 1868) and Fleming (Fleming, 1873), who discovered fluke eggs on sections of the “Delhi ulcer”. For the first time in print, the assumption about the bacterial nature of ulcers was made by Laveran in 1880 on the basis that ulcers more often appeared at the site of a violation of the integrity of the skin. Duclaux and Heidenreich (1885) isolated micrococcus from the blood of a patient and were inclined to consider it the causative agent. They named this micrococcus Micrococcus
biskra
, since the patient fell ill in Biskra. Similar observations were made by Chantemesse in a patient with Nile ulcer [4].
Many foreign researchers [4] still believe that the first (as mentioned above) to discover and describe the causative agent of cutaneous leishmaniasis was the Englishman Cunningham, who published his work in 1885. However, in this work, the detected microorganisms were not directly associated with the disease, but were accepted as contaminants.
In 1894 P.F. Borovsky began his work on the “Sartov ulcer,” as it was then called, and published the results of his research only in 1898, first in the form of a report at a congress of surgeons in Kyiv. In the same year, at a meeting of the Russian Surgical Society of Pirogov (protocol No. 162), he made a report “On the etiology of the Sartov (Eastern) ulcer,” accompanied by a demonstration of microscopic preparations. He chose a different path of research: “...In this case, we followed Rapchevsky’s plan and took for research cases of Sartov ulcer without any inflammatory phenomena.” At the very first search for P.F. Borovsky discovered a wide variety of airborne microflora that entered his crops, but assessed these findings as accidental. Smears from the juice of papules on glasses fixed in a flame “...gave a negative result in terms of the presence of microbes.” After this, Borovsky began to study the juice of papules, diluted with broth or saline in a hanging drop, and was struck by “... the presence of many round and irregular bodies.” Borovsky described in detail the bodies found. The size of the bodies in the fresh state ranges from 1.5 to 3 µ, rarely reaching 4 µ. “...In sections from compacted tissue they are less than 1-2 µ, but more than 1-1.5 µ.” The largest number of bodies is in fresh papules; as the ulcer ages, their number decreases; in old ulcers, Borovsky found these bodies only in isolated cases. The description of the pathogen stained with Leffler's solution is more reliable and contains all the main morphological elements characteristic of it. Borovsky described them as follows: “...Colored aliens appear for the most part in the form of round bodies with a sharply colored nucleus of variable shape - sometimes round, sometimes oblong, sometimes sickle-shaped - and weakly colored protoplasm; the nucleus is mostly located on the periphery, so that many corpuscles have a signet ring shape; aliens located in a cell push the cell nucleus to the periphery and are sometimes surrounded themselves, as it were, by a bag; in the latter case, the result is a cyst filled with aliens; Such cysts have also been observed outside cells.” Details of the structure of the parasite were also given: “Alien-eaters are observed, from the core of which there is a thin process to the opposite edge, and on this process there is a slight thickening.” Borovsky described the beginning of the division of the parasite with the division of the nucleus: “...Then (observed) such bodies were the nuclei were placed at opposite ends, so that there was an assumption about the preliminary division of the nucleus of the cell, and then the cell itself - the alien.” At the end of the report, Borovsky makes the main conclusion: “...Based on the structure of aliens, as well as on the basis of their properties to move and change shape, we are inclined to attribute them to Protozoa
", thereby expressing for the first time the idea of the protozoal nature of the causative agent of cutaneous leishmaniasis [4].
Borovsky’s main work, “On the Sartov Ulcer,” as mentioned above, appeared in 1898 in the Military Medical Journal. The general design of the article was the same as the report [4].
Borovsky gave an accurate description of the appearance of the parasite, discovered a nucleus in it, and definitely pointed to it as the nucleus of Protozoa
, noted the importance of this nucleus in the process of division of the parasite, described the vacuole; in his work there was a clear indication of the presence of a kinetoplast. The author showed that the causative agent of the oriental ulcer is an intracellular parasite; described spherical fragments of protoplasm with leishmania lying in them, proved that the parasite disappears from old ulcers and is replaced by ordinary wound microflora as recovery approaches, and revealed patterns of distribution of leishmania in the infiltrate. Finally, Borovsky was the first to publish the correct image of the pathogen. Thus, the doctrine of cutaneous leishmaniasis was first put on a truly scientific basis [4].
In 1903, resident of the Moscow Clinic of Skin Diseases S.L. Bogrov and microbiologist E.I. Martsinovsky, independently of P.F. Borovsky described the causative agent of cutaneous leishmaniasis. The authors found oval microorganisms in a patient with a “Persian ulcer”, which were given the name “ Ovoplasma
orientale
” and proved that these microorganisms belong to the phylum
Protozoa
(“On the question of the etiology of the oriental ulcer”, 1904) [5]. Thus, the study by E.I. Martsinovsky and S.L. Bogrov convincingly confirmed the discovery of P.F. Borovsky in 1898 [5, 6].
In 1907 Ch. Cassuto
in Tunisia isolated L. infantum
. In the same year E.I. Martsinovsky defended his dissertation on the etiology and clinical picture of leishmaniasis [3].
A significant milestone in the development of the doctrine of leishmaniasis was the work of G.I. Meshchersky [7] “Towards the clinic of cutaneous leishmaniasis”, published exactly a century ago - in 1915. The article provides an exhaustive description of the clinical picture of cutaneous leishmaniasis and for the first time noted a number of manifestations typical of this disease: the development of a tubercle as a primary element, then the formation of an ulcer, the presence of nodular lymphangitis (according to modern terminology - lymphangitis, N.S. Potekaev), which has a “cold” course, a pronounced tendency to “voluntary reverse development” and other signs [8].
In the same issue of the Russian Journal of Skin and Venereal Diseases, another work authored by S.L. is devoted to the problem of cutaneous leishmaniasis. Bogrov “On the anatomy of the eastern ulcer and accompanying nodular lymphangitis” (1915). The author proved that nodular lymphangitis is not a random complication of an eastern ulcer, as was previously thought, but is a specific component of this disease [8]. This resolved a long-standing debate among dermatologists about the nature of nodular lymphangitis in cutaneous leishmaniasis.
The works of V.L. were also of great importance in the study of leishmaniasis. Yakimova (1913), N.I. Khodukin and M.S. Sofieva (1929) [3].
Taxonomy
There are two main clinical and epidemiological variants of cutaneous leishmaniasis: anthroponotic (urban, late-ulcerating, dry cutaneous leishmaniasis) and zoonotic (rural, acute necrotizing, wet cutaneous leishmaniasis) types, the causative agents of which are considered as independent species. Among the local population, mainly children are affected; among visitors, people of any age are affected. In ICD-10, in the section “Protozoal diseases”, under code B.55, leishmaniasis is cutaneous, mucocutaneous, visceral, unspecified.
Pathogens belong to the phylum Sarcomastigophora
, subphylum
Mastigophora
, class
Zoomastigophorea
, order
Kinetoplastida
, suborder
Trypanosomatina
, family
Trypanosomatidae
, genus
Leishmania
2.
Leishmania is transmitted by mosquitoes3 ( Phlebotomus, Lutzomyia, Psychodopygus
, etc.). The infection season is associated with the summer period.
In the human body and other vertebrates, Leishmania has an intracellular non-motile leishmanial amastigote stage, and in the intestine of the carrier (phlebotomus) and in artificial media (at 25 °C) - a flagellated motile leptomonas stage (promastigote) [2].
In this regard, for the implementation of the transmission route, a necessary condition is a stable high ambient temperature (24–27 °C) for 3–4 weeks [9].
The source of pathogens for anthroponotic cutaneous leishmaniasis is a sick person, while for zoonotic leishmaniasis it is small mammals (gerbils).
At the site of entry of the pathogen, a granuloma develops - leishmanioma, which goes through the stage of incubation, proliferation (tubercle), destruction (ulcer) and repair (scarring). As a result of superinfection, several leishmaniomas can occur sequentially or simultaneously (with multiple bites). Along the lymphatic pathways from the primary focus to the regional lymph node, tubercles of contamination appear. The disease can acquire a chronic relapsing course in the form of small recurrent tubercles in the scar area (tuberculoid leishmaniasis).
Immunity to cutaneous leishmaniasis in humans is persistent and occurs only as a result of a natural or artificial (after vaccination) disease. Children born to immune parents remain susceptible to the process. Among the resident population of leishmaniasis-endemic foci, the disease mainly affects young children, as a result of which immunity is acquired in the first years of life. Therefore, cutaneous leishmaniasis is relatively rare among the adult resident population of these regions. Immunological changes in cutaneous leishmaniasis manifest themselves not only in the emergence of stable immunity, but also in the development of readiness for an allergic reaction to the introduction of a specific antigen [10]. Moreover, both types of cutaneous leishmaniasis are distinguished by the persistence and unity of immune reactions, which determines the high preventive effectiveness of cultural leishmania vaccinations (N.F. Rodyakin, 1957, 1982) [10].
Leishmaniasis cutaneous anthroponotic
has an incubation period of 2-8 months. Primary leishmanioma in the form of a smooth, slowly growing brownish-red tubercle reaches 1-2 cm in diameter after 6 months, then its surface begins to peel off and ulcerate after 5-10 months. Lumps of contamination may appear around the ulcer. After 1 year (sometimes more), the infiltrate decreases and the ulcer scars [9].
Leishmaniasis cutaneous zoonotic
characterized by a shorter incubation period (usually 1-4 weeks, but not more than 2 months), rapid growth and transformation of the tubercle into a boil-like element with a strong inflammatory infiltrate, unclear boundaries, and edema. The central part of the element quickly undergoes necrosis with the formation of a crater-shaped ulcer with purulent discharge. The process is accompanied by pain and the development of swelling of the feet and legs. After 3-6 months, the process ends with scarring [9]. Lymphangitis often occurs (defined as nodular, painless cords), lymphadenitis, which can ulcerate and scar [3]. The rural type of leishmaniasis is characterized by the frequent occurrence of additional tubercles around leishmaniamas as a result of dissemination of the pathogen [11].
The tuberculoid form described by I.I. Gitelzon (1932) and initially called metaleishmaniasis, develops after several months and sometimes years around the scar from former leishmaniomas, and is characterized by small dense tubercles of a yellowish-brown color, clinically similar to the manifestations of flat tuberculous lupus and giving the “apple jelly” symptom on diascopy . The tubercles are located in the form of rings, arcs, sometimes on the surface of the scar itself, and are not prone to ulceration. The process tends to last for a long time (many years) and slow peripheral growth [11]. Lepromatoid and intermediate forms of leishmaniasis have also been described. Diffuse infiltrating leishmanioma practically does not occur. The association of microbial flora complicates the course of leishmaniasis and delays recovery [3].
There are geographical types of the disease - leishmaniasis of the New and Old World. There are no fundamental differences in the pathogenesis and clinical picture of skin lesions. However, with the first, the ulcers are usually deeper (down to the hypodermis), sometimes accompanied by lymphangitis and lymphadenitis. The most important feature of cutaneous leishmaniasis of the New World is the frequent involvement of mucous membranes in the pathological process. As a rule, the mucous membranes are affected 1-2 years after the development of skin ulcers. Ulcerative-necrotic changes on the mucous membranes lead to deep deformation of the nose, ears, nasal pharynx, respiratory tract, genitals, disfiguring and disabling patients [3].
Several forms of New World cutaneous leishmaniasis are known: Mexican cutaneous leishmaniasis
(pathogen
L. mexicana
) with a benign but protracted course and rare damage to the mucous membranes
; Peruvian cutaneous leishmaniasis
(“uta”, the causative agent of
L. peruviana
) , which occurs mainly in children in dry and mountainous areas (the northern slopes of the Andes in Peru and Argentina, in Bolivia);
Venezuelan cutaneous leishmaniasis
(caused by
L. garnhami
), found in the highlands (800–1800 m above sea level) of western Venezuela in both rural and urban areas
; Guiana cutaneous leishmaniasis
(pathogen
L. guyanensis
the pathogen is bristly rats living in the forests of the northern regions of South America (Guyana, Panama, Suriname);
Panamanian cutaneous leishmaniasis
(caused by
L. panamensis
) is one of the most severe and common forms of cutaneous leishmaniasis in the New World, which is recorded in most countries of Central America [3] .
Diagnosis of the disease includes analysis of anamnestic, epidemiological, clinical data, laboratory test results: microscopy (detection of amastigotes in the contents of the edges of ulcers, impression smears, aspiration fluid from the infiltrate by staining according to Romanovsky-Giemsa, Wright), histological, cultural and parasitological diagnostics, molecular -biological methods (PCR), immunochromatographic express test rK39, biological test.
In zoonotic leishmaniasis, few leishmania are found in ulcers, so the search for the pathogen in the contents of ulcers, scrapings and skin biopsies should be more persistent [3].
Along with the classic clinical picture of cutaneous leishmaniasis, there are descriptions of not entirely typical clinical manifestations of the disease. A special place is occupied by chronic forms that occur as granulation inflammation. The article “Lymphocytoma-like leishmaniasis of the skin” (N.S. Potekaev, Yu.V. Postnov, N.N. Potekaev, Yu.P. Gribunov, A.N. Lvov, 2009) describes the observation of chronic skin leishmaniasis in a woman 40 years old (resident of Dagestan), which has been developing as a lymphocytoma for more than 15 years [12]. Repeatedly (including abroad) various nosologies were mistakenly assumed (eosinophilic granuloma, basal cell carcinoma, etc.), and only in 2006 the diagnosis of leishmaniasis was made first clinically by one of the authors of this article (N.S. Potekaev), and then confirmed pathohistologically (Yu.V. Postnov).
The work “Evolution of clinical manifestations of cutaneous leishmaniasis, leading to diagnostic errors” (D.D. Agakishiev, A.T. Gadzhieva, V.R. Guseinova, 2005) contains a description of the clinical observation of the leishmaniasis process in a 12-year-old girl, who, after staying in In an endemic zone in Azerbaijan, rashes appeared on the tip of the nose. The child received treatment for a diagnosis of pyoderma, and then for skin papilloma, but without effect. After a pathomorphological analysis of the lesion, an infiltrative form of cutaneous leishmaniasis was determined. The described case indicates that, despite the elimination of endemic foci of cutaneous leishmaniasis, sporadic cases of the disease occur [13]. From the point of view of differential diagnosis, the article “Leishmaniasis - a surprise from the East” in the journal “Child’s Health” (I.V. Bogadelnikov, Yu.V. Vyaltseva, I.Z. Karimov, A.A. Degtyareva, 2012) is interesting. The article presents a clinical case of cutaneous leishmaniasis in a 4-year-old child who came to Crimea from an area endemic for leishmaniasis - the Republic of Uzbekistan. The girl was repeatedly examined by pediatricians, surgeons, infectious disease specialists, and dermatologists, mistakenly assuming the presence of molluscum contagiosum. The diagnosis was confirmed by taking a smear from the ulcer and detecting Borovsky bodies [14]. In 2003 M.I. Kurdina described a case of tubercular leishmaniasis in a 16-year-old patient, a resident of the Moscow region, who became infected with urban-type leishmaniasis while on vacation in Cyprus. A rare tubercular form of leishmaniasis, tuberculoid, was diagnosed, and no leishmania was detected microscopically in scrapings from the lesions. The diagnosis was confirmed histologically by Prof. Yu.V. Postnov [15].
Differential diagnosis
carried out with tubercular syphilide, tuberculous lupus, chronic ulcerative pyoderma, boil (carbuncle), small nodular sarcoidosis, lupus erythematosus, tuberculoid type of leprosy, deep mycoses, various skin neoplasms.
Manifestations of skin leishmaniasis differ from tubercular syphilide by the localization of the rash mainly on open areas of the body, the lower density of the tubercles, the arrangement of elements on the scar, the positive “apple jelly” phenomenon, later ulceration, the nature of the scars (with syphilis the scar is mosaic, with leishmaniasis it is retracted), negative serological reactions to syphilis [16].
The manifestations differ from tuberculous lupus in that the process can occur at any age, and not primarily in childhood. The tuberculate elements are much denser, and therefore the phenomenon of probe failure is negative. The nature of the scar is also important: with tuberculous lupus the scar is superficial, with leishmaniasis it is deep, retracted [16]. However, “fresh” tuberculate elements can be located on the scar in both processes.
Cutaneous leishmaniasis also differs from chronic ulcerative pyoderma in the localization of rashes, multiplicity, rapid development of lymphangitis and lymphadenitis, the nature of the course of the skin process (leishmaniasis lasts on average up to a year, relapses of the disease are rare, while pyoderma proceeds for a long time, torpidly, with waves of exacerbations of the process ). Leishmanioma differs from a boil both in localization (the boil is present only in those areas of the skin where there are hair follicles) and in the absence of a purulent-necrotic core.
Leishmaniomas differ from rashes in small-nodular sarcoidosis in their tendency to ulceration (this is very rare in sarcoidosis), the formation of regional lymphadenitis, and a diascopic picture (the “dustiness” phenomenon in sarcoidosis).
Leishmaniasis differs from the tuberculoid type of leprosy in the absence of the tendency of leishmaniomas to group into shaped elements (as opposed to the tubercles in leprosy), the nature of the process (after 4-6 months, leishmaniomas ulcerate, and on average after 1 year the process ends with scarring, the course of leprosy is perennial), the absence sensitivity disorders and polyneuritis.
Treatment
The tactics of therapy depend on the form and stage of the disease, the general condition of the patient, and the type of pathogen. Rifampicin 600 mg per day is prescribed for 6 months, sometimes up to 1 year. A shorter dose of rifampicin 600 mg/day is possible - from 3 weeks to 3 months (sometimes longer) in combination with dapsone at a dose of 2 mg per 1 kg of body weight per day for 3 weeks. Against the background of systemic therapy, it is necessary to inject the lesions with pentavalent antimony preparations (glucantim, pentosta) 0.3-0.8 ml (total 5-6 ml per course). Injection is possible in combination with triamcinolone; is carried out every 2 days. The procedure is painful and requires a certain skill. When only puncturing the lesions, the clinical effect is not always sufficient. External dosage forms of pentavalent antimony (ointments, pastes) are ineffective in the treatment of tuberculoid cutaneous leishmaniasis [17].
In some cases, antimony preparations (pentostam, glucantim) are recommended to be administered intramuscularly or intravenously. The dose of the active substance for pentostam is calculated as follows: 10-20 mg per 1 kg of body weight per day (maximum 850 mg/day, or 8 ml of the drug), course duration - 20 days; or 20 mg/kg/day for 28 days. Cure is achieved in 80-90% of patients. Possible side effects: nausea, vomiting, myalgia, circulatory disorders, and therefore systemic treatment with antimony drugs is carried out in a specialized hospital. These effects are dose-dependent; if poorly tolerated, drugs are administered every other day [17].
There are reports of the effectiveness of ketoconazole at a dose of 400 mg/day for 3 months, or 600 mg/day for 28 days. Recently, itraconazole has been used at a dose of 200 mg/day (4 mg per 1 kg of body weight per day) for 1–3 months [17].
Other tested drugs - allopurinol, trichopolum, delagil - are much less effective. Data on their effectiveness are often contradictory and unreliable. As experimental schemes, puncture of lesions with recombinant interferon-γ is proposed [17].
We present the results of our observations.
Patient Z
., 53 years old, entered the Branch “Clinic named after. V.G. Korolenko" of the Moscow Scientific and Practical Center of Dermatovenereology and Cosmetology (MNPCDC) on November 8, 2013 with the diagnosis of the referring institution: "Microbial eczema." Upon admission: complaints of multiple rashes on the skin, accompanied by pain. From the anamnesis it became known that the patient considered herself sick since the end of September 2013, when she noted the appearance of the first rashes on the skin of the face, pain in the right arm, enlargement and tenderness of the lymph nodes. The rash appeared during the patient’s stay in rural areas of Uzbekistan (from 09/05/13). The expedition doctor regarded them as a manifestation of a pustular skin disease. She received treatment: doxycycline 100 mg 2 times a day for 5 days; gentamicin 0.4 2 times a day for 5 days; externally - levomekol ointment, synthomycin ointment, metrogil gel, without positive dynamics (new rashes appeared on the skin of the extremities, swelling of the hands). Since October 31, 2013, swelling of the tissues of the periorbital region on the left has developed. After returning to Moscow on November 5, 2013, swelling of the skin over the left ankle area developed (Fig. 1). She went to clinic No. 1 of the Ministry of Internal Affairs of Russia and was sent for inpatient treatment to the Branch “Clinic named after. V.G. Korolenko" MNPTSDC. Allergic history: angioedema is noted after the administration of novocaine, and a roseolous rash is observed after taking sulfonamides. Concomitant diseases: hypertension I-II degree, risk II. Obesity II degree. Chronic gastritis, remission. Chronic pancreatitis, remission. Anemia, mild course. Varicose veins of the lower extremities. Hiatal hernia. Retinal angiopathy. Presbyopia. OS - reactive edema.
Rice.
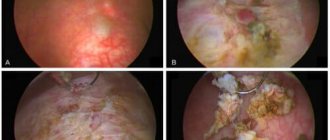
1. Dynamics of the skin process. Skin status at the time of admission: the pathological skin process is of an acutely inflammatory widespread nature, localized on the face (Fig. 2, a), auricle on the left (see Fig. 2, b), hands (see Fig. 2, c), c the area of the anterior surface of the right knee joint (see Fig. 2, d), in the area of the right shin in front and behind (see Fig. 2, e), in the area of the middle third of the left shin in front (see Fig. 2, f). It is represented by multiple round and infiltrated foci of bright red color ranging from 2 to 10 cm in diameter. In the center of some elements there are ulcerations covered with a crust. In the rashes there is a purulent component similar to boils. When you press on the tubercles, a yellowish discharge appears. When palpating the lesions, local pain is noted. Along the course of the lymphatic vessels, compactions in the form of ridges (rosaries) are determined.
Rice. 2. Patient Z., 53 years old. Rashes in the forehead (a), in the area of the auricle (b), in the area of the left hand (c), in the area of the anterior surface of the right knee joint (d), in the area of the shins (e), in the area of the middle third of the left shin in front ( e).
Laboratory examination data (11/19/13): clinical blood test showed ESR - 24 mm/h; indicators of clinical urine analysis, coagulograms are within normal limits; in a biochemical blood test, the level of total protein is 64 g/l; ECG data and chest x-ray showed no pathological changes; Ultrasound of the abdominal organs visualized signs of chronic cholecystitis and diffuse changes in the pancreas; When analyzing the discharge from the lesions (11/18/13), leishmania was detected. In a preparation from the contents of an ulcer dated November 18, 2013, stained according to Romanovsky under direct microscopy (UV 1000), oval bodies were found in the cytoplasm of macrophages, as well as freely lying ones, the cytoplasm of which was colored grayish-blue. The nuclei are pushed to the periphery, their color is somewhat darker. In the cytoplasm, the kinetoplast is clearly visible - a rod-shaped formation, stained more intensely than the nucleus (Fig. 3). Conclusion: Leishmania, a form of amastigotes, was detected.
Rice. 3. Preparation of phagocytosed leishmania (Uv. 1000). Romanovsky-Giemsa staining. 1 - phagocyte with the inclusion of Leishmania; 2 - Leishmania (Borovsky bodies); 3 - kinetoplast.
Based on the anamnestic data, clinical picture, and positive analysis of leishmania discharge from the lesions, a diagnosis was made: “Zoonotic type of cutaneous leishmaniasis.”
On November 28, 2013, the patient was consulted by a parasitologist at CDC No. 1 of the First Moscow State Medical University. THEM. Sechenov Prof. N.I. Tumolskaya, who confirmed the diagnosis of cutaneous leishmaniasis (form L.
m ajor
).
In the hospital, treatment was carried out with available antibacterial drugs (ceftriaxone 1.0 2 times a day intramuscularly; gentamicin 0.8 2 times a day intramuscularly; doxycycline 100 mg 2 times a day orally), antifungal drugs (fluconazole 50 mg/day). day, orungamine 200 mg/day); antiprotozoal drugs (metrogyl 100.0 intravenous drip); aminoquinoline drugs (plaquenil 200 mg 2 times a day); other classes of drugs (methyluracil 500 mg 2 times a day, Essentiale 300 mg 3 times a day, xyzal 5 mg 1 time a day, sorbifer 100 mg 2 times a day). Pure ichthyol and 3% boric acid were prescribed externally.
The parasitologist prescribed the following treatment: glucantim at the rate of 60 mg per 1 kg of body weight per day. Divide the dose as follows: on the 1st day - 1 ampoule intramuscularly, on the 2nd day - 2 ampoules intramuscularly deep, on the 3rd day - 3 ampoules intramuscularly, on the 4th day - a full dose of 5 ampoules (25 ml) . Course 10-15 days (not fully effective against L. major
).
Paromimycin 0.5 3 times a day for 10-15 days (specific drug for L. major
).
A pronounced positive trend was obtained. After transmitting information about the diagnosis in accordance with the provisions of Order No. 20/9 dated January 13, 2004 of the Moscow Department of Health and the Center for State Sanitary and Epidemiological Surveillance in Moscow “On the procedure for special recording of infectious and parasitic diseases in Moscow,” the patient was transferred under the supervision of a specialist for further treatment. parasitologist
In conclusion, it should be noted that anti-epidemic and preventive measures in foci of zoonotic cutaneous leishmaniasis are much more complex and less effective than in anthroponotic leishmaniasis, and depend on the structure of the foci, the type of the predominant reservoir of infection, and the state of the natural biocenosis in the area. The basis of all activities is the widespread use of all methods of exterminating wild desert rodents. As a preventive measure, vaccination is used, which is carried out in the autumn-winter season (but no later than 3 months before departure to an area endemic for zoonotic cutaneous leishmaniasis), as a result of which stable lifelong immunity develops [3]. In areas with a hot climate, everyone outdoors should use special mosquito nets, which in this case are personal protective equipment, as well as repellents. It is impossible to overestimate the timeliness of detection and treatment of all cases, which makes it possible to prevent the spread of cutaneous leishmaniasis among the population.
In 1950-1970 In the USSR, targeted work was carried out to combat leishmaniasis, as a result of which the incidence of some forms (anthroponotic cutaneous, urban form of visceral leishmaniasis) was practically eliminated. However, our previously published work convincingly demonstrated the possibility of infection with anthroponotic leishmaniasis in Dagestan [8].
It is assumed that Leishmania has existed as part of the trypanosomatid family for about 35–25 million years, i.e., it appeared even before the separation of the continents [3].
The idea of the possibility of the spread of leishmaniasis by phlebotomuses was expressed by Pressot (1905), but its experimental confirmation was obtained later. The zoonotic nature of one of the forms of cutaneous leishmaniasis was established by N.N. Latyshev et al. (1941) [3].