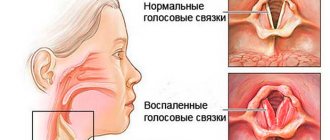
Ipraterol is a bronchodilator solution for inhalation in a nebulizer, which relieves shortness of breath, spasm, and makes it possible to breathe more freely in asthma, COPD, bronchitis, as well as diseases of the throat and larynx accompanied by spastic phenomena. It also comes in an aerosol can.
For 1 inhalation, an adult needs 1-2 ml of liquid, a child 6-12 years old needs 0.5-1 ml, they are diluted with saline to obtain 3-4 ml of the composition. Ipraterol is contraindicated at the beginning and end of pregnancy, during breastfeeding, certain heart problems, and allergies. Sometimes he should prefer Berodual, even though it is more expensive. The solution also has direct analogues: Inspirax, Astmasol, Fenipra.
Features of the action
Ipraterol inhaler contains several active components with antispasmodic action - ipratropium bromide monohydrate, fenoterol hydrobromide. They enhance and complement each other's influence, accelerating the therapeutic effect. When ingested through a nebulizer, relief occurs within 5 minutes. The maximum effect is observed after 2 hours. The effect lasts up to 6 hours. If the dosage is exceeded or used incorrectly, undesirable effects occur. Inhalation of Ipraterol for cough is done after consultation with a doctor, determining the exact dosage.
Composition and release forms
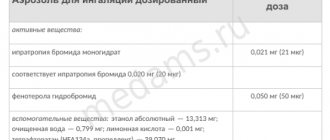
Ipraterol for inhalation therapy consists of 2 main substances: bromide and hydrobromide. The auxiliary component is sodium edetate. Ipraterol is produced in 2 forms:
- inhalation solution;
- drops in bottles.
The drug Ipraterol Aeronative is available in the form of a metered aerosol, which is used for inhalation. The drug is convenient to use. Inhalation with Ipraterol does not require preliminary preparation, since the product is available in a can with a valve and a spray nozzle. This packaging provides aerosol spraying of Ipraterol.
Application
According to the instructions, the drug is prescribed for diseases of the respiratory system with obvious signs of dysfunction of the lungs and bronchi. The medication has a pronounced antispasmodic effect on smooth muscles, relaxes, and improves breathing. The product acts locally and, when used correctly, does not affect the cardiac, nervous, or circulatory system.
The main indications according to the instructions are:
- asthma;
- severe bronchitis;
- COPD
Ipraterol Nativ for inhalation is used for the treatment and prevention of asthma attacks when exposed to provoking factors.
Useful video
Possible side effects
Ipraterol Nativ when inhaled can provoke adverse reactions, including tremor, nervousness, increased heart rate, and arrhythmia.
On a note! Taking the medication affects the mucous membrane of the respiratory system, simultaneously causing dryness in the mouth. Tachycardia, urticaria and vision problems are common symptoms of overdose.
Nervousness is one of the adverse reactions when using the drug
Contraindications
According to the instructions for use, the medication should absolutely not be prescribed if there is an individual intolerance to the components or severe pathologies of the cardiac or circulatory system. Inhalations are not prescribed in the first stages of pregnancy. When breastfeeding, the baby is transferred to artificial nutrition for the duration of therapy.
Relative contraindications according to the instructions are associated with disruption of the heart, circulatory system, genitourinary system, blood vessels, and organs of vision. For children under six years of age, Ipraterol Nativ inhalation solution is used under the strict supervision of specialists.
Special instructions
If shortness of breath (difficulty breathing) suddenly increases rapidly, you should consult a doctor immediately.
In children, the drug should be used only as prescribed by a doctor and under the supervision of adults.
In athletes, the use of the drug Ipraterol-native due to the presence of fenoterol in its composition can lead to positive results of doping tests.
Patients should be instructed on the correct use of Ipraterol-native inhalation solution. To prevent the solution from getting into the eyes, it is recommended that the solution used with a nebulizer be inhaled through the mouthpiece. If there is no mouthpiece, a mask that fits tightly to the face should be used. Patients predisposed to developing glaucoma should take special care to protect their eyes.
No studies have been conducted to study the effect of the drug on the ability to drive vehicles and operate machinery. Cases of dizziness and blurred vision while using the drug may have a negative impact on the above-mentioned ability.
How to inhale with Ipraterol Nativ
According to the instructions, the medication is prescribed for acute attacks of suffocation, severe coughing, and for preventive purposes. The procedures are carried out once when a spasm occurs or undergo a course of treatment. The dosage and duration of therapy are selected by a specialist.
For long-term use, it is recommended to do the procedures according to the instructions 1-2 times a day on an empty stomach. Food in the stomach reduces the therapeutic effect. In emergency situations, the medication is administered at any time, regardless of food intake. After breathing procedures, you cannot go outside in any weather or eat for 2 hours.
Pharmacological properties
Pharmacodynamics
Ipraterol-native is a bronchodilator drug containing two components with bronchodilator activity: an m-anticholinergic blocker - ipratropium bromide and a selective beta2-adrenergic agonist - fenoterol.
The bronchodilator effect of the Ipraterol-native inhalation solution is achieved as a result of the action of the m-anticholinergic blocker and beta2-adrenergic agonist on various pharmacological targets. By complementing each other's effect, the active ingredients enhance the antispasmodic effect on the bronchial muscles. The combined composition provides a wide range of therapeutic effects of the drug for bronchopulmonary pathologies accompanied by constriction of the airways, allowing the desired effect to be achieved using a lower dose of the beta-adrenergic component. The combination of ipratropium bromide and fenoterol allows you to select an individual effective dose, significantly reducing the risk of side effects.
Due to the fact that the effect of the drug in the treatment of acute bronchoconstriction develops quickly, it is effectively used to treat acute attacks of bronchospasm.
Ipratropium bromide
Ipratropium bromide is a quaternary ammonium derivative with a pronounced anticholinergic (parasympatholytic) effect. The substance has a depressant effect on the reflexes caused by the vagus nerve, thereby counteracting the acetylcholine mediator released from the endings of the vagus nerve. Anticholinergics prevent the increase in the intracellular level of calcium ions, which occurs as a result of the interaction of acetylcholine and muscarinic receptors of bronchial smooth muscle. The release of calcium ions indirectly involves a system of secondary mediators, including ITP (inositol triphosphate) and DAG (diacylglycerol).
When administered by inhalation, bronchodilation is largely due not to the systemic, but to the local anticholinergic effect of ipratropium bromide.
After inhalation, a significant improvement in lung function in patients with bronchospasm associated with chronic obstructive pulmonary pathologies (including chronic bronchitis, pulmonary emphysema) occurs within 1/4 hour. There is an increase of 15% or more in forced expiratory volume in 1 second and peak expiratory flow. The maximum effect is achieved after 1-2 hours and lasts for 6 hours in most patients.
Ipratropium bromide does not adversely affect mucociliary clearance, gas exchange and mucus secretion in the respiratory tract.
Fenoterol hydrobromide
Fenoterol – 1-(3,5-Dioxyphenyl)-2-(para-hydroxy-a-methyl-phenethylamino)-ethanol, beta2-adrenergic receptor agonist. In therapeutic doses, fenoterol stimulates beta2-adrenergic receptors selectively, and its stimulating effect on beta1-adrenergic receptors appears only when using high doses.
The relaxing effect of fenoterol on the smooth muscles of the bronchi and blood vessels helps prevent the development of bronchospastic reactions that occur under the influence of methacholine, histamine, allergens and cold air. Its blocking effect on the release of inflammatory mediators and bronchial obstruction from mast cells occurs immediately after use. With the use of higher doses of fenoterol, an increase in mucociliary clearance is observed.
The vascular effect of fenoterol, stimulating beta2-adrenergic receptors of the heart, determines the beta-adrenergic effect of the drug on cardiac activity, causing an increase in the frequency and strength of heart contractions. Like other beta-adrenergic agents, high doses of fenoterol may cause QTc prolongation. The clinical significance of this manifestation has not been established.
The most common side effect of beta-agonists is tremor.
Pharmacokinetics
Ipratropium bromide, when administered by inhalation, is extremely poorly absorbed from the mucous membrane of the respiratory tract. Its plasma concentration can only be determined using special enrichment methods or high doses. The total systemic bioavailability of the substance after inhalation ranges from 7 to 28%. Due to the use of ipratropium bromide in therapeutic doses, its plasma concentrations are 1000 times lower compared to this value after intravenous or oral administration.
When fenoterol is inhaled, 10–30% reaches the lower respiratory tract (depending on the inhalation method and the inhalation system used), the rest is swallowed and enters the gastrointestinal tract. There is no correlation between fenoterol plasma levels achieved after inhalation and the pharmacodynamic time-effect curve. The duration of the bronchodilator effect is not maintained in the systemic circulation by high concentrations of the active substance. After fenoterol is administered orally, about 60% of the dose is absorbed; it takes 2 hours to reach its maximum concentration (Cmax) in the blood plasma.
Being a derivative of quaternary nitrogen, ipratropium bromide is poorly soluble in fatty media and is not capable of significant penetration through biological membranes and does not accumulate.
The binding of fenoterol to plasma proteins is 40–55%. Fenoterol crosses the placental barrier unchanged and is excreted in breast milk.
Ipratropium bromide is metabolized in the liver to form eight known ipratropium metabolites. Their connection with muscarinic receptors is insignificant.
Fenoterol is metabolized in the liver. Its biotransformation occurs in the intestinal wall exclusively through conjugation with sulfates. The ingested amount of the drug after inhalation has virtually no effect on the level of the active substance in the blood plasma.
Excretion of ipratropium bromide occurs mainly through the intestines: unchanged - about 25%, in the form of metabolites - the rest.
Fenoterol is excreted through the kidneys and intestines in the form of inactive sulfate conjugates.
The pharmacokinetic parameters of a drug containing a combination of ipratropium bromide and fenoterol in elderly and children, patients with impaired liver and/or kidney function, and patients with diabetes mellitus have not been studied.
Inhalation of Ipraterol for children
According to the instructions, a new solution is prepared before the event. The duration of inhalations is 5-15 minutes, depending on the clinical picture. According to the instructions, the drug is not prescribed to children under six years of age, but in critical cases the medication is used. The amount of medication is determined based on body weight.
| Age | Quantity for acute spasms in ml, drops for small children | Moderate situation (ml) | Severe attacks (ml) |
| From 6 to 12 years | 0,5 | 1 | 2 |
| Under 6 years old | 2 k. | 5 k. | 10 k. |
The maximum dose for children under 12 years of age is 3 ml or 60 drops. Inhalation is done under the supervision of doctors. During long-term treatment, breathing procedures are performed up to 4 times a day. No more than 80 drops are administered per day. The maximum dosage for children under 6 years of age is 1.5 ml or 10 drops.
Treatment begins with a minimum amount of active substances. If there is no therapeutic effect, the dosage is increased. Any nebulizer is used for inhalation. You need to breathe until there is no medicine left in the inhaler chamber.
Analogs
A large group of drugs have a similar principle of biochemical action to Ipraterol-native or similar active substances.
| Name | Active substances | Diseases |
| Berodual | fenoterol hydrobromide ipratropium bromide | Asthma, suffocating cough of unknown etiology, bronchial hypersensitivity, chronic obstructive bronchitis, allergic asthma. |
| Seretide | fluticasone propionate salmeterol xinafoate | Bronchial asthma, maintenance therapy for COPD. |
| Salbutamol | Salbutamol sulfate | Relieves and prevents asthmatic attacks, bronchospasm |
| Pulmicort | budesonide | bronchial asthma, COPD. |
| Berotek | fenoterol hydrobromide | Bronchial asthma, narrowing of the airways, emphysema, as a prevention of asthma as a result of physical activity. |
| Atrovent | Ipratropium bromide | Severe form of bronchial inflammation, bronchospasms, bronchial asthma in combination with cardiovascular diseases. |
| Foradil | formoterol fumarate is a selective beta 2-adrenergic receptor agonist | Dilates the bronchi with irreversible airway obstruction, prevents swelling and accumulation of inflammatory cells. |
| Tevacombe | salmeterol, fluticasone propionate | Relieves bronchospasm in the case of maintenance therapy for COPD. |
Many of the above products are available not only in the form of solutions for inhalation, but also aerosols. Despite the similar principle of action of drugs, a doctor must prescribe and select the dose.
Ipraterol Nativ solution for adults
The instructions for use are the same, the dosage of the drug is different.
- For acute attacks, adults are administered 1 ml of the drug or 20 drops.
- Increase the dose to 2.5 ml for severe attacks. 50 drops are dropped into the nebulizer.
- The maximum dosage is 4 ml, per day – 8 ml or 160 drops.
For long-term therapy, 1-2 ml of Nativ is administered 4 times a day. As you feel better, reduce the dosage. The minimum amount for adults is 1.5 ml.
Overdose
- symptoms: the most likely symptoms, usually associated with the action of fenoterol and caused by excessive stimulation of β-adrenergic receptors, include the appearance of tremor, increased blood pressure, increased gap between SBP and DBP, tachycardia, palpitations, angina pectoris, arrhythmias, flushing of the face, a feeling of heaviness in the chest, increased metabolic acidosis, bronchial obstruction. Due to an overdose of ipratropium bromide, a slight disturbance of eye accommodation and dry mouth may occur; taking into account the breadth of the therapeutic effect and the local method of application of the substance, they are usually mild and transient;
- treatment: the use of Ipraterol-native inhalation solution must be stopped, after which, taking into account data from monitoring the acid-base balance of the blood, sedatives and tranquilizers (anxiolytics) are prescribed. Severe cases of overdose may require intensive therapy to treat. Selective beta1-blockers can be used as a specific antidote. In this case, the dose of the beta-blocker should be carefully selected, taking into account the possible increase in bronchial obstruction in patients with chronic obstructive pulmonary disease or bronchial asthma, which can lead to the development of severe bronchospasm, even death.
Drug interactions
When used simultaneously with Ipraterol-native:
- other anticholinergic drugs: their prolonged simultaneous use with Ipraterol-native is not recommended due to the lack of data;
- cromoglycic acid, glucocorticosteroids: their additional administration helps to increase the effectiveness of therapy;
- beta-adrenergic agonists, systemic anticholinergics, theophylline and other xanthine derivatives: concomitant therapy with these drugs may enhance the bronchodilator effect of the solution and lead to exacerbation of adverse reactions;
- beta-blockers: can significantly weaken the bronchodilator effect of Ipraterol-native;
- diuretics, xanthine derivatives, glucocorticosteroids: the possible increase in existing hypokalemia with this combination should be taken into account, especially in patients with severe forms of obstructive respiratory diseases; with this combination, monitoring of serum potassium concentration is recommended;
- Digoxin: In patients with hypokalemia, digoxin may increase the risk of arrhythmias. If it is necessary to take digoxin, regular monitoring of serum potassium levels is required;
- monoamine oxidase inhibitors (MAO), tricyclic antidepressants: caution should be exercised when combined with Ipraterol-native due to increased beta-adrenergic action;
- halothane, trichlorethylene, enflurane: general anesthesia with inhalation of halogenated hydrocarbon anesthetics may increase the effect of the drug on the cardiovascular system.
What diseases is it used to treat?
Ipraterol Aeronative solution is prescribed to patients in the following situations:
- treatment of obstructive type respiratory tract pathologies - these include lung diseases, bronchial-type asthma, bronchitis, which occurs in an advanced form and is accompanied by emphysema),
- prevention of the above diseases.
Inhalations are required only after a doctor’s indication - otherwise the medication may have a negative effect on the patient’s health, as well as aggravate the ongoing pathology.