Beclazon
Before prescribing inhaled drugs, it is necessary to instruct the patient about the rules for using the drug to ensure the most complete delivery of the drug to the desired areas of the lungs.
Not intended for the relief of acute asthmatic attacks. Patients should be aware of the preventive nature of the drug and that to achieve optimal effect, the inhaler should be used regularly, even in the absence of asthma symptoms.
With regular inhalations, improvement in breathing usually occurs after 1 week of treatment. Lack of effect is possible in patients with increased levels of sputum and mucus in the respiratory tract and severe bronchospasm, which prevents the drug from reaching the zone of action. In such cases, inhalation of adrenergic stimulants is prescribed 15-30 minutes before inhalation of beclomethasone or treatment is started with the systemic use of GCS.
The transfer of patients regularly taking oral corticosteroids to inhaled beclomethasone, as well as subsequent treatment, should be carried out with extreme caution, under daily monitoring of peak flowmetry (pneumotachometry), since suppression of the adrenal cortex caused by long-term use of corticosteroids is restored slowly.
Before prescribing inhaled forms of beclomethasone, patients should be in a relatively stable condition, and their prescription itself should complement the usual maintenance dose of systemic corticosteroids. After about 1 week, the daily dose of steroids begins to be gradually reduced - 1 mg/week (in terms of prednisolone). Deterioration of the condition against the background of a maintenance dose of 400 mcg/day means the need to transfer patients to oral prednisolone. Regular use allows in most cases to cancel oral corticosteroids (patients who need to take no more than 15 mg of prednisolone can be completely transferred to inhalation therapy), while in the first months after the transition the patient’s condition should be carefully monitored until his pituitary-adrenal system recover sufficiently to respond to stressful situations (such as injury, surgery, or infection).
Patients transferred to inhalation treatment and having impaired function of the adrenal cortex should have a supply of GCS and with them a warning card, which should indicate that in stressful situations they need additional systemic administration of GCS (after eliminating the stressful situation, the dose of steroids can be repeated reduce). Sometimes a transfer from taking systemic corticosteroids to inhalation administration can lead to the manifestation of previously suppressed forms of allergies, such as allergic rhinitis or eczema.
It is necessary to protect the eyes from contact with the drug.
It is advisable to rinse the mouth and throat after inhalation (prevention of candidiasis), and when initial signs of fungal infection of the oral mucosa appear, use nystatin, fluconazole, amphotericin. By washing after inhalation you can prevent damage to the skin of the eyelids and nose.
The maximum daily dose of the drug in adults should not exceed 1 mg. At a dose of up to 1.5 mg/day in most patients, it does not significantly suppress adrenal function. If this dose is exceeded, some patients may experience some suppression of adrenal function. Treatment in doses of more than 1 mg/day is carried out under the supervision of a physician.
During pregnancy and breastfeeding, the drug should be used with caution and only if the benefit from its use outweighs the potential risk. There is insufficient data on the safety of beclomethasone in pregnant women and its excretion in women's breast milk.
Beclazone preparations, containing 50-100 mcg in 1 dose, play an important role in the treatment of severe forms of bronchial asthma in children, since they provide good control over the course of the disease and do not cause growth retardation in the child. The drug at a dose of 250 mcg is not intended for use in pediatrics. It is recommended to regularly monitor the growth dynamics of children receiving inhaled corticosteroids over a long period of time.
Infectious and inflammatory diseases of the respiratory system are not a specific contraindication for treatment with beclomethasone.
The drug should not be frozen or exposed to direct sunlight. The can cannot be punctured, disassembled or thrown into fire, even if it is empty. When the canister cools, it is recommended to remove it from the plastic case and warm it with your hands (at low temperatures, the effectiveness of the drug is reduced).
Is it possible to use Beclazon IVF 250 during pregnancy and lactation?
Beclazone IVF 250 mcg in one dose (as well as 100 and 50 mcg) is used with caution during pregnancy. In the first trimester they try not to use it, and from the second it is prescribed only when the threat to the mother’s health is higher than the risks to the development of the fetus. The hormone passes into breast milk, so it is most often recommended to transfer the baby to formula milk during lactation. This is mandatory when using doses above the minimum (from 200 mcg).
Analogues of Beclazon
Beclazon ECO has complete analogues in composition and release form:
- Beclomethasone Aeronative “Pharmstandard”, Russia;
- Beclomethasone "Binnopharm", Russia.
Beclomethasone Aeronative "Pharmstandard" Beclomethasone "Binnopharm" Clenil Sabacomb Nasobek Foster
They are cheaper than the imported drug. Beclomethasone is also available in the form of a nasal spray (Nasobek), an inhalation suspension (Klenil), and is part of combined antiasthmatic drugs (Foster, Sabacomb).
Directions for use and dosage
At the beginning of treatment, the dose of Beclazone is selected individually by the doctor, who takes into account the severity of the disease. When treating bronchial asthma, children over 12 years of age and adults are usually prescribed:
- For mild cases – 200 mcg 2 times a day;
- For moderate severity – 600-800 mcg per day, divided into 2-4 doses;
- In severe cases, a maximum dose of 1 mg can be used, which is also distributed over 2-4 inhalations.
The initial dose of Beclazon for children 4-12 years old is 50-100 mcg 2 times a day, if necessary, the dose can be increased to 400 mcg, divided into 2-4 inhalations. The maximum daily recommended dose for children is 500 mcg.
After using the drug, you should rinse your mouth with water to avoid a sore or irritated throat.
Beclazone, if necessary, can be used simultaneously with sodium cromoglycate, bronchodilators and antibiotics.
Overdose
An acute overdose of Beclazone Eco can develop with inhalation of a single dose of more than 1000 mg. Symptoms of suppressed function of the adrenal cortex do not require emergency treatment, since the disorder goes away within a few days.
In case of chronic overdose (long-term therapy at a dose of more than 1500 mg), persistent suppression of adrenal function may develop. In this case, monitoring of the reserve function of the adrenal cortex is indicated.
Treatment with beclomethasone in case of overdose can be continued in doses that are sufficient to maintain the therapeutic effect.
Indications for use
The active ingredient of the drug has a pronounced anti-allergic, anti-inflammatory and anti-exudative effect. As a result of the use of Beclazone, the secretion of mucus by the bronchial glands and swelling of the epithelium are reduced, and indicators of external respiration function are improved.
It is recommended to use the drug regularly, regardless of attacks. The therapeutic effect of the drug develops gradually, improvement usually occurs within a week after the start of therapy.
Beclazon is prescribed:
- Prevention of exacerbations of bronchial asthma and its treatment when therapy with bronchodilators and sodium cromoglycate in combination with bronchodilators is ineffective;
- Treatment of hormone-dependent bronchial asthma.
The drug does not relieve bronchospasm.
Release form and composition
The dosage form of Beclazone Eco is a dosed aerosol for inhalation: placed under pressure in an aluminum can; there must be no external damage, leaks or corrosion; when sprayed onto glass, the contents of the can leave a white spot (in a cardboard pack there is 1 bottle of 200 doses).
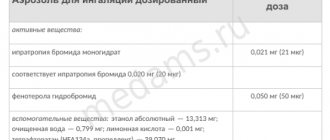
Composition of 1 dose:
- active substance: beclomethasone dipropionate – 0.05 mg, 0.1 mg or 0.25 mg;
- auxiliary components (0.05/0.1/0.25 mg): ethanol – 2.09/3.11/6 mg; hydrofluoroalkane (HFA-134a) – 75.86/74.79/71.75 mg.
Pharmacological properties
Pharmacodynamics
Beclomethasone is a GCS (glucocorticosteroid). It has a weak affinity for GCS receptors.
Under the influence of enzymes, it is converted into an active metabolite - B-17-MP (beclomethasone-17-monopropionate), which has a pronounced local anti-inflammatory effect.
Main properties of Beclazon Eco:
- reduction of inflammation by reducing the formation of chemotaxis substance (impact on late allergy reactions);
- inhibition of the development of an immediate allergic reaction (associated with inhibition of the production of arachidonic acid metabolites and a decrease in the release of inflammatory mediators from mast cells);
- improvement of mucociliary transport;
- decrease in the number of mast cells in the bronchial mucosa;
- reduction of epithelial edema, mucus secretion by bronchial glands, bronchial hyperreactivity, marginal accumulation of neutrophils, inflammatory exudate and production of lymphokines;
- inhibition of macrophage migration;
- reducing the intensity of granulation and infiltration processes;
- increase in the number of active beta-adrenergic receptors;
- restoration of the patient’s response to bronchodilators, which allows reducing the frequency of their use.
Beclomethasone after inhalation has virtually no resorptive effect.
Beclazon Eco does not relieve bronchospasm. The development of the therapeutic effect occurs gradually, usually after 5–7 days of course use.
Pharmacokinetics
More than 1/4 of the dose of inhaled beclomethasone settles in the respiratory tract, the rest settles in the mouth, pharynx and is swallowed. In the lungs, beclomethasone is intensively metabolized to the active metabolite B-17-MP before absorption.
Systemic absorption of the B-17-MP metabolite occurs in the lungs (36% of the pulmonary fraction) and in the gastrointestinal tract (26% of the ingested dose).
The absolute bioavailability of unchanged beclomethasone and B-17-MP is approximately 2 and 62% of the inhalation dose, respectively. Beclomethasone is rapidly absorbed, Tmax (time to reach maximum concentration in blood plasma) is 18 minutes. The absorption of B-17-MP is slow, its Tmax is 60 minutes.
There is a linear relationship between the increase in the dose received and the systemic exposure of the substance.
Distribution in tissues of beclomethasone and B-17-MP is 20 and 424 l, respectively. There is a relatively high connection with blood plasma proteins - 87%.
Beclomethasone and B-17-MP have high plasma clearance (150 and 120 l/h, respectively). The half-life is 0.5 and 2.7 hours, respectively.
Side effects
Possible adverse reactions (> 10% - very common; > 1% and < 10% - often; > 0.1% and < 1% - uncommon; > 0.01% and < 0.1% - rare; < 0. 01%, including individual messages - very rarely):
- endocrine system (with long-term use of doses of more than 1500 mg per day): very rarely - inhibition of the HPA axis (hypothalamic-pituitary-adrenal system);
- respiratory system: often – hoarseness, irritation of the pharyngeal mucosa; rarely – paradoxical bronchospasm; very rarely - eosinophilic pneumonia;
- musculoskeletal system: very rarely - decreased bone mineral density;
- organ of vision: very rarely – glaucoma, cataract;
- allergic reactions: rarely - angioedema, erythema, itching, skin rash, urticaria;
- infectious diseases (with prolonged use in doses of 0.4 mg per day): often - candidiasis of the oral cavity, pharynx and upper respiratory tract;
- systemic reactions: nausea, headache, thinning of the skin or bruising.
Is it possible to overdose Beclazon IVF with beclomethasone?
If the maximum doses (1-2 mg) of Belazon IVF with beclomethasone are used several times, this can lead to a temporary decrease in the functioning of the adrenal glands. After discontinuation of the drug, the function is restored within a few days.
If bronchial asthma is severe, and large dosages must be used frequently, then there is a stable suppression of hormonal synthesis by the adrenal glands. Therefore, it is important to regularly monitor urinary cortisol levels and adjust the dose of Beclazone.
Interaction of Beclazone aerosol with other drugs
The main adverse interactions between Beclazone aerosol and medications are listed in the table.
| Name of drug or group | Result of interaction |
| Salbutamol, Berotec, Foradil, Clenbuterol, female sex hormones, Prednisolone, Dexamethasone | The effectiveness of Beclazone increases |
| Insulin, glucose-lowering tablets | There is a risk of increased glucose levels |
| Aspirin | Possibility of gastric damage, erosion, ulcers |
| Diuretics | Their effectiveness decreases |
| Antiviral | Increases the risk of side effects of Beclazone |
| Rifampicin | Reduces reaction to Beclazone |