Release form and composition
Ketorolac is available in the following dosage forms:
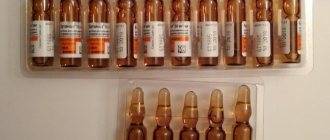
- Solution for intravenous and intramuscular administration: yellowish, transparent (1 ml in ampoules, 5, 10 ampoules in pallets, 1 or 2 pallets in a cardboard box; 2 ml in dark glass ampoules, 10 ampoules in a cardboard box) ;
- Solution for intramuscular administration: transparent, light yellow (1 ml in ampoules, 5 ampoules in pallets, 1 or 2 pallets in a cardboard box, or 5 or 10 ampoules in a cardboard box; 2 ml in dark glass ampoules, 10 ampoules in a cardboard box);
- Tablets (10 pcs. in blister packs, 1, 2, 5, 10 packs in a cardboard pack; 20 pcs. in blister packs, 1 pack in a cardboard pack; 25 pcs. in blister packs, according to 2, 4 packages in a cardboard box; 10 pieces in polymer cans, 1 can in a cardboard box);
- Film-coated tablets: white, biconvex (10 pieces in blister packs, 1-3, 5 packs in a cardboard box);
- Film-coated tablets: biconvex, round, almost white or white; on the cross-section two layers are visible (10 pieces in strip packs, 1-5, 10 packs in a cardboard box; 20, 100 pieces in cans, 1 can in a cardboard box).
The composition of 1 ml of solution for intravenous and intramuscular administration includes:
- Active substance: ketorolac trometamol – 30 mg (ketorolac tromethamine);
- Auxiliary components: sodium chloride, disodium edetate (ethylenediamine-N,N,N',N'-tetraacetic acid disodium salt 2-aqueous (Trilon B)), water for injection.
The composition of 1 ml of solution for intramuscular administration includes:
- Active substance: ketorolac trometamol – 30 mg;
- Auxiliary components: disodium edetate dihydrate (Trilon B), ethanol (ethyl alcohol in terms of anhydrous), propylene glycol, sodium chloride, sodium hydroxide (1M solution), water for injection.
1 tablet contains the active substance: ketorolac tromethamine – 10 mg.
The composition of 1 film-coated tablet includes:
- Active substance: ketorolac trometamol – 10 mg (ketorolac tromethamine);
- Auxiliary components: lactose, potato starch, microcrystalline cellulose, calcium stearate, Opadry II.
The composition of 1 film-coated tablet includes:
- Active substance: ketorolac trometamol – 10 mg (ketorolac tromethamine);
- Auxiliary components: microcrystalline cellulose, magnesium stearate, talc, crospovidone (kollidon CL), milk sugar (lactose monohydrate).
Shell composition: hydroxypropyl methylcellulose (hypromellose), titanium dioxide, talc, propylene glycol, macrogol 4000 (polyethylene oxide 4000, polyethylene glycol 4000).
Ketorolac
Active substance:
Ketorolac*
Pharmgroup:
NSAIDs. Acetic acid derivatives and related compounds
Average price in pharmacies
| Name | Manufacturer | average price |
| Ketorolac 0.01 n14 tablet p/cap/coat /update/ | UPDATE | 39.00 |
| Ketorolac 0.01 n20 tablet p/o/Borisov ZMP/ | Borisov Medical Preparations Plant, JSC | 35.00 |
| Ketorolac 0.01 n20 tablet p/film/coating/synthesis | SYNTHESIS | 35.00 |
| Ketorolac 0.01 n20 tablet p/plen/envelop/tathimpharm | TATHIMPHARMA PREPARATIONS | 30.99 |
| Ketorolac 0.03/ml 1ml n10 amp solution i.v. i.m./biosynthesis | Biosynthesis of PAO | 60.00 |
Analogs for the active substance:Adolor Dolak Dolomin Ketalgin Ketanov Ketolak Ketorol Ketorolac Rompharm Ketorolac-OBL Ketorolac-Eskom Ketorolac tromethamine Ketofril Toradol Thorolak | Application area:Obstetric and gynecological pain Pain syndrome Pain syndrome in the back area Pain syndrome in oncological practice Pain syndrome in the postoperative period Pain syndrome in the postoperative period Pain syndrome in the postoperative period after orthopedic surgery Pain syndrome in the postoperative period after orthopedic surgery Pain syndrome of inflammatory origin Severe pain syndrome Pain syndrome of non-oncological origin Pain syndrome of non-rheumatic origin Pain syndrome after diagnostic procedures Pain syndrome after diagnostic procedures Pain syndrome after diagnostic interventions Pain syndrome after diagnostic interventions Pain syndrome after surgery Pain syndrome after surgery Pain syndrome after surgical interventions Pain syndrome after surgical interventions Pain syndrome after orthopedic surgery Pain syndrome after orthopedic surgery Pain syndrome after injuries Pain syndrome after injuries Pain syndrome after removal of hemorrhoids Pain syndrome after removal of hemorrhoids Pain syndrome after surgery Pain syndrome after surgery Pain syndrome with vertebrogenic lesions Pain syndrome due to inflammation of non-rheumatic nature Pain syndrome in inflammatory lesions of the peripheral nervous system Pain syndrome in diabetic neuropathy Pain syndrome in malignant neoplasms Pain syndrome in muscular and joint diseases Pain syndrome with neuralgia Pain syndrome with neuralgia Pain syndrome from burns Pain syndrome in cancer Pain syndrome with tumors Pain syndrome in acute inflammatory diseases of the musculoskeletal system Pain syndrome due to tendon pathology Pain syndrome when using excimer laser Pain syndrome with radiculitis Pain syndrome with radiculitis Pain syndrome due to smooth muscle spasms Pain syndrome due to smooth muscle spasms (renal and biliary colic, intestinal spasms, dysmenorrhea) Pain syndrome due to spasms of smooth muscles of internal organs Pain syndrome due to spasms of smooth muscles of internal organs (renal and biliary colic, intestinal spasms, dysmenorrhea) Pain syndrome due to injuries Pain syndrome due to injuries Pain syndrome during injuries and after surgery Pain syndrome during injuries and after surgery Pain syndrome during injuries and after surgery Pain syndrome in chronic inflammatory diseases of the musculoskeletal system Pain syndrome in chronic inflammatory diseases of the musculoskeletal system Pain syndrome in duodenal ulcer Pain syndrome with gastric ulcer Pain syndrome in gastric and duodenal ulcers Pain syndrome is mild or moderate Pain syndrome in cancer patients Painful sensations Pain in the muscles Pain during menstruation Pain syndromes Pain syndromes in dental practice Painful conditions Painful tired legs Sore gums when wearing dentures Muscle soreness Muscle soreness during heavy physical activity Tenderness of cranial nerve exit points Painful diagnostic interventions Painful diagnostic procedures Painful instrumental diagnostic procedures Painful instrumental manipulations Painful treatment procedures Painful manipulations Painful, irregular periods Painful dressings |
Contraindications
- Rhinitis, urticaria caused by taking non-steroidal anti-inflammatory drugs (history);
- Complete or partial combination of recurrent polyposis of the paranasal sinuses and nose and bronchial asthma with intolerance to acetylsalicylic acid or other non-steroidal anti-inflammatory drugs (including a history);
- Dehydration, hypovolemia (regardless of the cause that caused it);
- Intolerance to pyrazolone drugs;
- Bleeding, high risk of its occurrence;
- Confirmed hyperkalemia;
- Condition after coronary artery bypass surgery;
- Erosive and ulcerative lesions of the gastrointestinal tract (with exacerbation), peptic ulcers;
- Inflammatory bowel diseases;
- Hypocoagulation (including hemophilia);
- Severe renal failure (with creatinine clearance less than 30 ml per minute);
- Hemorrhagic stroke (suspected or confirmed);
- Active liver disease or severe liver failure;
- Hematopoietic disorder;
- Hemorrhagic diathesis;
- Age up to 16 years (the effectiveness and safety of the drug for this age group of patients have not been established);
- Pregnancy and breastfeeding (lactation);
- Childbirth;
- Hypersensitivity to the components of the drug and other non-steroidal anti-inflammatory drugs.
The drug should not be used to treat chronic pain.
Due to the high risk of bleeding, Ketorolac is not used as a means for maintenance anesthesia, premedication, or pain relief before and during surgical operations (including obstetric practice).
Ketorolac should be used with caution in the following diseases/conditions:
- Arterial hypertension;
- Bronchial asthma;
- Chronic heart failure;
- Cholecystitis;
- Functional kidney disorders (with plasma creatinine below 50 mg/l);
- Cholestasis;
- Sepsis;
- Active hepatitis;
- Systemic lupus erythematosus;
- Polyps of the mucous membrane of the nasopharynx and nose, simultaneous use with other non-steroidal anti-inflammatory drugs;
- Cerebrovascular diseases;
- The presence of factors that increase gastrointestinal toxicity, including smoking and alcoholism;
- Edema syndrome;
- Postoperative period;
- Hyperlipidemia/dyslipidemia;
- Cardiac ischemia;
- Peripheral arterial diseases;
- Diabetes;
- Creatinine clearance is less than 60 ml per minute;
- History of ulcerative lesions of the gastrointestinal tract;
- Helicobacter pylori infection;
- Severe somatic diseases;
- Old age (over 65 years old);
- Long-term use of non-steroidal anti-inflammatory drugs;
- Concomitant use of oral glucocorticosteroids (including prednisolone), antiplatelet agents (including clopidogrel), anticoagulants (including warfarin), selective serotonin reuptake inhibitors (including citalopram, paroxetine, fluoxetine, sertraline).
Main characteristics
Ketorolac (Ketanov, Ranbaxy Pharmaceuticals Ukraine LLC (SAN PHARMA group of companies)) is an NSAID with powerful analgesic activity. Based on the results of a study of ketorolac on animals, it was found that ketorolac is 800 times more effective than acetylsalicylic acid. A study by C. Brown et al proved that intramuscular administration of 30 mg of ketorolac has an effect comparable to the effect of 10–12 mg of morphine or 50 mg of meperidine.
In terms of analgesic activity, ketorolac is superior to most NSAIDs, such as diclofenac, ibuprofen, ketoprofen and many others; it also has moderate anti-inflammatory properties and has an antipyretic effect.
The drug does not affect opioid receptors, does not have a sedative or anxiolytic effect, does not suppress the activity of the respiratory center, does not cause euphoria, drug dependence and spasm of the smooth muscles of internal organs.
Ketorolac in standard doses and for short-term use is quite safe, which makes it the drug of choice for the relief of acute pain in therapeutic practice. It can be successfully used for acute pain in the lower back, toothache, migraine, renal and biliary colic, and in many other situations when fast and powerful pain relief is required, and the use of narcotic analgesics is impractical.
Ketorolac has been shown to be an effective drug for the treatment of renal colic, does not require close patient monitoring, and does not cause adverse sedation.
A number of clinical cases have shown that intravenous administration of 30 mg of ketorolac over 10–20 minutes significantly relieves pain in renal colic and the results of administration of one dose of ketorolac are superior to the results of administration of a similar dose of the narcotic analgesic meperidine.
When prescribing various NSAIDs (metamizole sodium, diclofenac, ketorolac, paracetamol) to patients in therapeutic departments and surgical practice, it was concluded that ketorolac is the best drug for emergency pain relief in terms of efficacy/safety ratio and subjective assessment of the effectiveness of pain relief (by visual analogue). scale - YOURS).
In addition, adequate relief of pain with ketorolac is an important additional factor in stabilizing blood pressure in patients with hypertensive crisis.
Based on the data obtained, the authors classified ketorolac as an effective and safe medicine used in the complex treatment of diseases of internal organs (pleuropneumonia, chronic non-calculous cholecystitis, urolithiasis, etc.) complicated by pain.
The question of the true incidence of serious complications that occur while taking ketorolac remains open. Specialists studying the effectiveness and safety of drugs necessarily take into account the preclinical data provided by the manufacturer, however, in the process of large-scale clinical use of any drug, new quantitative and qualitative data on adverse reactions appear, which are analyzed and make it possible to assess the true safety profile only after many years and even decades .
The interest in ketorolac is largely determined by the fact that it was first tested as a parenteral analgesic in the USA, a country that not only maintains priority in analgesic therapy, but also has a guaranteed multi-stage system for testing the effectiveness and safety of drugs.
Possible undesirable effects of ketorolac may be associated with the effect of NSAIDs on blood clotting. According to the results of a retro- and prospective study conducted on the basis of clinical anesthesiology, intensive care and emergency medicine of FPO, ketorolac is a safe and effective analgesic NSAID that does not have a significant effect on the blood coagulation system, platelet aggregation with a single use for the purpose of premedication during anesthesia /operations.
This study was one of the stages of an open-label, multicenter, prospective study using retrospective data of the safety profile of Ketanov (ketorolac tromethamine), carried out at the initiative of the drug manufacturer Ranbaxy Laboratories Ltd (India).
Thus, an analysis of retrospective data from studies from 2000–2004. indicates that a summary assessment of the characteristics of side effects in 790 surgical patients and 429 dental patients revealed the development of expected non-serious adverse reactions in only 2.4% of them, the development of which did not require discontinuation of the drug or adjustment of the dosage regimen.
Thus, taking into account world, European and Russian recommendations for the treatment of pain syndromes, ketorolac is a modern analgesic that provides a sufficient effect for pain of any intensity, and its widespread use both in outpatient clinics, emergency care, and in multidisciplinary hospitals is justified from the standpoint of evidence medicine.
For severe pain requiring complex analgesic therapy, ketorolac is an excellent addition to narcotic analgesics, as well as local or regional anesthesia. The drug can be used either once or for longer (up to 5–7 days) treatment.
The availability of an injection solution and a tablet form of the drug allows one to significantly optimize treatment regimens for patients with pain of moderate and high intensity, including through stepwise therapy.
If necessary, the patient can take it at home independently. Ketorolac is well tolerated and has a low incidence of side effects. Limiting the duration of treatment to achieve a positive effect can be a reliable way to increase the safety of therapy.
Directions for use and dosage
Ketorolac in tablet form is taken orally.
Depending on the severity of the pain syndrome, the drug is used once (at a dose of 10 mg) or repeatedly (10 mg up to 4 times a day).
The maximum daily dose is 40 mg.
Ketorolac solution is administered deeply intramuscularly or intravenously (in a stream) slowly (at least 15 seconds) in the smallest effective doses, which are selected depending on the intensity of pain and the patient’s response. If necessary, simultaneous use with opioid analgesics in reduced doses is possible.
Depending on the severity of the pain syndrome, the adult single dose for a single administration (intramuscular or intravenous) is 10-30 mg. Patients over 65 years of age or with functional renal impairment are usually prescribed 10-15 mg.
For repeated parenteral administration of Ketorolac, the following dosage regimens are used:
- Intramuscular administration: adults under 65 years of age and children over 16 years of age - 10-60 mg for the first injection, then every 6 hours 10-30 mg (usually 30 mg); elderly patients (over 65 years old) or with functional renal impairment - 10-15 mg every 4-6 hours;
- Intravenous administration: adults under 65 years of age and children over 16 years of age - 10-30 mg as a bolus for the first injection, then every 6 hours at 10-30 mg, the initial dose for continuous infusion using an infusion pump is 30 mg, then the infusion rate is 5 mg per hour; elderly patients (over 65 years old) or with functional renal impairment - 10-15 mg infusion every 6 hours.
The maximum daily dose of Ketorolac for intramuscular and intravenous administration is:
- Adults under 65 years of age and children over 16 years of age – 90 mg;
- Elderly patients (over 65 years old) or with functional renal impairment - 60 mg.
Continuous intravenous infusion should not last longer than 24 hours.
The duration of the course when taking Ketorolac orally and parenterally should not be longer than 5 days.
When switching from intramuscular and intravenous administration to taking Ketorolac orally, the total daily dose on the day of transfer should not exceed:
- Adults under 65 years of age and children over 16 years of age – 90 mg;
- Elderly patients (over 65 years old) or with functional renal impairment - 60 mg.
Moreover, on the day of transition, the dose of the drug in tablet form should not exceed 30 mg.
Pharmacodynamics and pharmacokinetics
Pharmacodynamics
It has a strong analgesic effect, moderate antipyretic and anti-inflammatory effect.
They are associated with indiscriminate inhibition of the enzyme cyclooxygenase of the first and second types in peripheral tissues, resulting in inhibition of the synthesis of prostaglandins - mediators of pain, inflammation and thermoregulation.
The drug has no effect on opioid receptors, does not cause addiction, does not depress breathing, and does not have a sedative or anxiolytic effect.
The strength of the analgesic effect is comparable to morphine and superior to other drugs in its group.
After oral administration, the analgesic effect is recorded after one hour, the greatest effect – after one to two hours. After an intramuscular injection, the onset of the analgesic effect is recorded after 30 minutes, the greatest effect – after one to two hours.
Pharmacokinetics
When taken internally and when administered by injection, it is actively absorbed from the intestines and tissues. The maximum concentration in the blood is recorded after 40-50 minutes, both after oral administration and after intramuscular injection. Eating does not affect absorption. Plasma protein binding is about 99%.
The half-life is approximately 6 hours. 90% of the dose is excreted by the kidneys, in its original form - 60%; the remaining amount is excreted through the digestive tract.
Side effects
During the use of Ketorolac, it is possible to develop disorders of certain body systems, manifesting themselves with varying frequencies (often - >3%; less often - 1-3%; rarely - <1%):
- Central and peripheral nervous system: often – drowsiness, dizziness, headache; rarely - aseptic meningitis (including convulsions, fever, severe headache, stiffness of the back and/or neck muscles), hyperactivity (including anxiety, mood changes), depression, hallucinations, psychosis;
- Respiratory system: rarely - dyspnea, bronchospasm, pulmonary edema, rhinitis, laryngeal edema (including shortness of breath, difficulty breathing);
- Cardiovascular system: less often - increased blood pressure; rarely - fainting;
- Digestive system: often (especially in patients over 65 years of age with a history of erosive and ulcerative lesions of the gastrointestinal tract) - diarrhea, gastralgia; less often - flatulence, stomatitis, vomiting, constipation, feeling of fullness in the stomach; rarely - nausea, loss of appetite, erosive and ulcerative lesions of the gastrointestinal tract (including with bleeding and/or perforation - melena, abdominal pain, burning or spasm in the epigastric region, nausea, vomiting with blood or coffee-like vomiting thick, heartburn), hepatomegaly, cholestatic jaundice, acute pancreatitis, hepatitis;
- Blood coagulation system: rarely - rectal and nasal bleeding, bleeding from a postoperative wound;
- Hematopoietic system: rarely – eosinophilia, anemia, leukopenia;
- Urinary system: rarely - lower back pain, acute renal failure, azotemia, hematuria, hemolytic-uremic syndrome (renal failure, hemolytic anemia, purpura, thrombocytopenia), increased or decreased urine volume, frequent urination, edema of renal origin, nephritis;
- Skin: less often – purpura, skin rash (including maculopapular); rarely - exfoliative dermatitis (including fever with or without chills, redness, peeling or thickening of the skin, tenderness and/or swelling of the tonsils), Lyell's syndrome, Stevens-Johnson syndrome, urticaria;
- Sense organs: rarely - ringing in the ears, hearing loss, visual impairment (including blurred vision);
- Local reactions: less often - pain or burning at the injection site;
- Allergic reactions: rarely - anaphylactoid reactions or anaphylaxis (including changes in facial skin color, skin rash, urticaria, dyspnea or tachypnea, skin itching, periorbital edema, swelling of the eyelids, difficulty breathing, shortness of breath, wheezing, heaviness in the chest);
- Other: often - weight gain, swelling (including ankles, face, fingers, legs, feet); less often – increased sweating; rarely – fever, swelling of the tongue.
Features of the use of the medicine
Ketorolac in tablet form is taken orally. Depending on the severity of the pain syndrome, the drug is used once (at a dose of 10 mg) or repeatedly (10 mg up to 4 times a day). The maximum daily dose is 40 mg.
Ketorolac solution is administered deeply intramuscularly or intravenously (in a stream) slowly (at least 15 seconds) in the smallest effective doses, which are selected depending on the intensity of pain and the patient’s response. If necessary, simultaneous use with opioid analgesics in reduced doses is possible.
Indications
Ketorolac tablets are prescribed for moderate to severe pain, including injuries, toothache, pain in the postoperative period, cancer, myalgia, neuralgia, arthralgia, sciatica, rheumatic diseases, sprains, dislocations.
Ketorolac has no effect on the progression of the disease. The drug should be used only for symptomatic treatment, reducing inflammation and pain at the time of use.
Contraindications for ketorolac
The drug should not be used to treat chronic pain. Due to the high risk of bleeding, Ketorolac is not used as a means for maintenance anesthesia, premedication, or pain relief before and during surgical operations (including obstetric practice).
Contraindications to the use of the drug are:
- complete or incomplete combination of bronchial asthma, recurrent polyposis of the nose and paranasal sinuses and intolerance to acetylsalicylic acid or other NSAIDs (including a history);
- urticaria, rhinitis caused by taking NSAIDs (history);
- intolerance to pyrazolone drugs;
- dehydration, hypovolemia (regardless of the cause);
- bleeding or a high risk of developing it;
- condition after coronary artery bypass surgery;
- confirmed hyperkalemia;
- inflammatory bowel diseases;
- erosive and ulcerative lesions of the gastrointestinal tract in the acute stage, peptic ulcers;
- hypocoagulation (including hemophilia);
- severe renal failure (creatinine clearance less than 30 ml/min);
- severe liver failure or active liver disease;
- hemorrhagic stroke (confirmed or suspected);
- hemorrhagic diathesis;
- hematopoietic disorder;
- pregnancy;
- childbirth;
- lactation period (breastfeeding);
- children under 16 years of age (efficacy and safety have not been established);
- hypersensitivity to ketorolac and other NSAIDs.
The drug is not used as a means for premedication, maintenance of anesthesia, pain relief before and during surgical operations (including in obstetric practice) due to the high risk of bleeding. The drug is not indicated for the treatment of chronic pain.
With caution: bronchial asthma; cholecystitis; chronic heart failure; arterial hypertension; impaired renal function (plasma creatinine below 50 mg/l); cholestasis; active hepatitis; sepsis; systemic lupus erythematosus; old age (over 65 years).
Polyps of the mucous membrane of the nose and nasopharynx, simultaneous use with other NSAIDs; the presence of factors that increase gastrointestinal toxicity: alcoholism, smoking; postoperative period, edema syndrome, coronary artery disease, cerebrovascular diseases, dyslipidemia/hyperlipidemia, diabetes mellitus, peripheral arterial diseases.
Creatinine clearance less than 60 ml/min, a history of ulcerative lesions of the gastrointestinal tract, the presence of Helicobacter pylori infection, long-term use of NSAIDs, severe somatic diseases, simultaneous use of oral corticosteroids (including prednisolone), anticoagulants (including warfarin), antiplatelet agents (including clopidogrel), selective serotonin reuptake inhibitors (including citalopram, fluoxetine, paroxetine, sertraline).
Dosage
For oral administration. Ketorolac should be used orally once or repeatedly depending on the severity of the pain syndrome.
A single dose is 10 mg; when taken again, it is recommended to take 10 mg up to 4 times a day, depending on the severity of pain. The maximum daily dose should not exceed 40 mg. When taken orally, the duration of the course should not exceed 5 days.
Parenterally The drug should be administered deep IM or IV (stream) slowly for at least 15 seconds in the minimum effective doses, selected in accordance with the intensity of pain and the patient’s response. If necessary, additional opioid analgesics can be prescribed at the same time in reduced doses.
Doses for single parenteral administration. Single doses for a single intramuscular or intravenous injection: adults under 65 years of age - 10–30 mg, depending on the severity of the pain syndrome; for elderly patients over 65 years of age or with impaired renal function - 10–15 mg.
Doses for repeated parenteral administration. For intramuscular administration, adults under 65 years of age and children over 16 years of age are administered intramuscularly with 10–60 mg for the first injection, then 10–30 mg every 6 hours (usually 30 mg every 6 hours); Elderly patients over 65 years of age or with impaired renal function - 10-15 mg every 4-6 hours.
For intravenous administration, adults under 65 years of age and children over 16 years of age are injected in a dose of 10–30 mg, then 10–30 mg every 6 hours. For continuous infusion using an infusion pump, the initial dose is 30 mg, then the infusion rate is 5 mg/h.
When administered intravenously to elderly patients over 65 years of age or with impaired renal function, a dose of 10–15 mg is administered bolus every 6 hours.
The maximum daily dose for intramuscular and intravenous administration for adults under 65 years of age and children over 16 years of age is 90 mg; for elderly patients over 65 years of age or with impaired renal function - 60 mg.
Continuous IV infusion should not last more than 24 hours. When administered parenterally, the duration of the course of treatment should not exceed 5 days.
When switching from parenteral administration of the drug to oral administration, the total daily dose of the drug in both dosage forms on the day of transfer should not exceed: 90 mg for adults under 65 years of age and children over 16 years of age and 60 mg for elderly patients over 65 years of age or with impaired renal function. In this case, the dose of the drug in tablets on the day of transition should not exceed 30 mg.
Side effects
Often - more than 3%, less often - 1-3%, rarely - less than 1%. From the digestive system: often - gastralgia, diarrhea; less often - stomatitis, flatulence, constipation, vomiting, feeling of fullness in the stomach; rarely - loss of appetite, nausea, erosive and ulcerative lesions of the gastrointestinal tract (including with perforation and/or bleeding - abdominal pain, spasm or burning in the epigastric region, blood in the stool or melena, vomiting with blood or coffee type thick", nausea, heartburn, etc.), cholestatic jaundice, hepatitis, hepatomegaly, acute pancreatitis.
From the urinary system: rarely - acute renal failure, lower back pain, hematuria, azotemia, hemolytic-uremic syndrome (hemolytic anemia, renal failure, thrombocytopenia, purpura), increased urination, increased or decreased urine volume, nephritis, edema of renal origin.
From the senses: rarely - hearing loss, ringing in the ears, visual impairment (including blurred visual perception). From the respiratory system: rarely - bronchospasm or shortness of breath, rhinitis, pulmonary edema, laryngeal edema (shortness of breath, difficulty breathing).
From the side of the central nervous system: often - headache, dizziness, drowsiness; rarely - aseptic meningitis (fever, severe headache, convulsions, stiffness of the neck and/or back muscles), hyperactivity (mood changes, anxiety), hallucinations, depression, psychosis, fainting.
From the cardiovascular system: less often - increased blood pressure. From the hematopoietic organs: rarely - anemia, eosinophilia, leukopenia. From the hemostasis system: rarely - bleeding from a postoperative wound, nosebleeds, rectal bleeding.
From the skin: less often - skin rash (including maculopapular rash), purpura, rarely - exfoliative dermatitis (fever with or without chills, flushing, thickening or peeling of the skin, enlargement and/or tenderness of the tonsils), urticaria, malignant exudative erythema (Stevens-Johnson syndrome), toxic epidermal necrolysis (Lyell's syndrome).
Local reactions: less often - burning or pain at the injection site. Allergic reactions: rarely - anaphylaxis or anaphylactoid reactions (change in facial skin color, skin rash, urticaria, itching of the skin, tachypnea or shortness of breath, swelling of the eyelids, periorbital edema, shortness of breath, difficulty breathing, heaviness in the chest, wheezing).
Other: often - swelling (face, legs, ankles, fingers, feet, weight gain); less often - increased sweating, rarely - swelling of the tongue, fever.
special instructions
When Ketorolac is used simultaneously with other nonsteroidal anti-inflammatory drugs, fluid retention, the development of cardiac decompensation, and arterial hypertension may occur.
To reduce the risk of developing gastropathy associated with the use of non-steroidal anti-inflammatory drugs, misoprostol, antacids, and omeprazole are prescribed.
The effect on platelet aggregation after using Ketorolac lasts for 24-48 hours.
Hypovolemia increases the risk of developing renal side effects.
Ketorolac should not be used simultaneously with paracetamol for more than 5 days.
For patients with bleeding disorders, the drug is prescribed only with constant monitoring of the platelet count, especially in the postoperative periods, which require careful monitoring of hemostasis.
Due to the fact that a significant proportion of patients during therapy develop side effects from the central nervous system (headache, dizziness, drowsiness), performing work that requires high attention and quick reactions (working with machinery, driving vehicles) is not recommended.
Indications for use
Moderate to severe pain syndrome :
- toothache;
- pain of traumatic etiology;
- pain in the postoperative and postpartum period;
- pain due to cancer ;
- dislocations , sprains ;
- arthralgia , neuralgia , myalgia , radiculitis ;
- rheumatic diseases.
Used for symptomatic therapy, relief of inflammation and pain at the time of use, does not affect the development of the disease.
Drug interactions
When Ketorolac is used simultaneously with certain medications, undesirable effects may occur:
- Acetylsalicylic acid or other non-steroidal anti-inflammatory drugs, glucocorticosteroids, calcium preparations, ethanol, corticotropin: formation of gastrointestinal ulcers and development of gastrointestinal bleeding;
- Paracetamol: increased nephrotoxicity;
- Methotrexate: increased hepato- and nephrotoxicity (simultaneous use is possible only when used in low doses while monitoring plasma concentrations);
- Methotrexate, lithium: decreased clearance and increased toxicity;
- Indirect anticoagulants, heparin, thrombolytics, antiplatelet agents, cefoperazone, cefotetan and pentoxifylline: increased risk of bleeding;
- Probenecid and drugs that block tubular secretion: decreased clearance of Ketorolac and increased its concentration in the blood plasma;
- Antihypertensive and diuretic drugs: decreased effect;
- Opioid analgesics: enhancing their effect (doses can be significantly reduced);
- Insulin and oral hypoglycemic drugs: increased hypoglycemic effect (dose recalculation required);
- Valproic acid: impaired platelet aggregation;
- Verapamil, nifedipine: increasing their concentration in blood plasma;
- Other nephrotoxic drugs (including gold preparations): increased risk of nephrotoxicity;
- Myelotoxic drugs: increased manifestation of hematotoxicity of the drug.
Ketorolac in the form of an injection solution should not be mixed in the same syringe with morphine sulfate, hydroxyzine and promethazine (due to precipitation).
Ketorolac is pharmaceutically incompatible with lithium preparations and tramadol solution; compatible with 5% dextrose (glucose) solution, 0.9% sodium chloride solution, Plasmalit solution, Ringer-lactate and Ringer's solution, as well as infusion solutions containing aminophylline, lidocaine hydrochloride, dopamine hydrochloride, short-acting human insulin and heparin sodium salt.