| Clomipramine | |
| Clomipraminum | |
| Chemical compound | |
| IUPAC | 3-(3-chloro-10,11-dihydro-5 H -dibenzo[ b, f ]azepin-5-yl)- N,N -dimethylpropan-1-amine |
| Gross formula | C19H23ClN2 |
| Molar mass | 314,9 |
| CAS | 303-49-1 |
| PubChem | 2801 |
| DrugBank | APRD00253 |
| Classification | |
| ATX | N06AA04 |
| Pharmacokinetics | |
| Bioavailable | Approximately 50% when taken orally |
| Metabolism | Liver |
| Half-life | Clomipramine about 35 hours Desmethylclomipramine (the most active metabolite) about 50 hours |
| Excretion | Kidneys |
| Dosage forms | |
| tablets and dragees 10 and 25 mg, extended-release tablets 75 mg, ampoules 2 ml of 2.5% solution (25 mg) | |
| Methods of administration | |
| orally, intramuscularly, intravenously | |
| Other names | |
| Anafranil, Gidifen, Clominal, Clofranil, Maronil, Hydiphen, Klomipramin, Monochlorimipramine, Neoprex | |
| Media files on Wikimedia Commons | |
Clomipramine
is a tricyclic antidepressant, similar in action to imipramine, but more powerful in blocking the reuptake of serotonin. It also has α-adrenergic blocking and antihistamine effects. It has a pronounced thymoleptic effect with a weaker stimulating component than imipramine. It differs in structure from imipramine only in the presence of a chlorine atom in the molecule.
Carefully
Clomipramine is used with caution in patients with heart disease, a history of epilepsy, breastfeeding, the elderly, liver failure, thyroid disease, pheochromocytoma, a history of psychosis (clomipramine may increase psychotic symptoms), a history of urinary retention, while undergoing electroconvulsive therapy , simultaneous anesthesia (increases the risk of arrhythmias and arterial hypotension)[3].
The use of clomipramine during pregnancy, especially in the first and third trimesters, should be avoided unless absolutely necessary[3].
When a woman takes clomipramine during breastfeeding, side effects may occur in the infant; The infant should be monitored for lethargy and drowsiness[3].
It should be taken into account that clomipramine may impair the ability to control moving machinery and impair the ability to drive a car[3].
Clomipramine should not be discontinued abruptly[3].
Release form and composition
- Film-coated tablets (10 pieces in blisters, 2 or 3 blisters in a cardboard box);
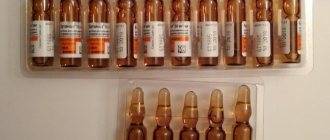
- Solution for intramuscular and intravenous administration (in ampoules of 2 ml, in a cardboard box of 10 ampoules).
The active ingredient of Anafranil is clomipramine.
Auxiliary components in the tablets: lactose, stearic acid, corn starch, magnesium stearate, colloidal anhydrous silicon dioxide, talc, titanium dioxide, glycerin 85%, vinylpyrrolidone/vinyl acetate copolymer, crystalline sucrose, hydroxypropyl methylcellulose, PVP K30, MCC, iron oxide yellow 5 %, titanium dioxide 95%, polyethylene glycol 8000.
Excipients in the solution: water for injection, glycerol.
Side effects
The main side effects of clomipramine are anticholinergic effects[2] (accommodation disorders, constipation, urinary retention, dry mouth, confusion[2], increased intraocular pressure[3]), orthostatic hypotension, and less commonly cardiotoxic effects (ECG monitoring is required)[2] . Cardiotoxic side effects of clomipramine include ECG changes, arrhythmias, orthostatic hypotension, and tachycardia[3]. Unlike imipramine, clomipramine does not generally increase fear and anxiety. Like SSRI antidepressants, it can lead to the development of sexual dysfunction[4].
Possible dyspepsia[5], nausea, sedation, insomnia, agitation[6], behavioral disorders[3], agitation, exacerbation of paranoid symptoms, phase inversion (i.e. development of mania or hypomania), ataxia, convulsions, dyskinesias, muscle twitching[5 ] (especially in elderly patients), tremors, fainting, increased sweating, rash and hypersensitivity reactions (urticaria, photosensitivity), sexual dysfunction, changes in blood sugar levels, endocrine side effects (testicular enlargement, gynecomastia, galactorrhea), increased appetite and increased body weight (sometimes weight loss), fever, agranulocytosis, leukopenia, eosinophilia, purpura, thrombocytopenia, hyponatremia, changes in liver function tests[3].
Anticholinergic syndrome may occur when taking clomipramine[7]. When clomipramine is combined with some other antidepressants (MAOI drugs[8], SSRI drugs[9]), and sometimes with clomipramine monotherapy[10], the development of serotonin syndrome[8][9][10] is possible. In cases where clomipramine is used for liver failure, increased sedation is possible [3].
In cases of intravenous drip administration of clomipramine, careful monitoring of blood pressure should be carried out, since orthostatic collapse in these cases is frequent, especially in the first hours after infusion therapy[2].
Instructions for use of Anafranil: method and dosage
Anafranil is taken orally, after or during meals, or administered intramuscularly or intravenously.
To treat phobias, depression, and obsessive-compulsive disorders, take Anafranil orally 2-3 times a day, 25 mg.
During the first week, the daily dosage of the drug is gradually increased to 100-150 mg. After the condition improves, the patient is transferred to maintenance therapy - 50-100 mg per day.
The drug is administered intramuscularly at a dosage of 25-50 mg, after which the dose is increased every day by 25 mg, bringing it to 100-150 mg/day. After the condition has stabilized, the number of injections is reduced, and the patient is transferred to taking the drug in tablets.
50-75 mg of Anafranil is administered intravenously over 1.5-3 hours. A single infusion is carried out; before administration, the drug is dissolved in dextrose or sodium chloride solution. After the desired effect is achieved, the drug is continued for another 3-5 days.
As a maintenance treatment, the patient is prescribed Anafranil tablets.
For the treatment of narcolepsy, the drug is prescribed in a daily dosage of 25-75 mg, orally.
To relieve chronic pain, take tablets in a dosage of 10-150 mg/day.
For attacks of fear, take Anafranil at a dosage of 10 mg/day.
Elderly patients are prescribed the drug at a dosage of 10 mg/day, gradually increasing the dose to 30-50 mg.
For children, the drug is prescribed at a dose of 10 mg and increased over 10 days:
- For children 5-7 years old – up to 20 mg;
- For children 8-14 years old – up to 20-50 mg;
- For children over 14 years old – up to 50 mg and above.
Interactions
The drug is not combined with irreversible MAOIs, reserpine[5]. When combined with MAOIs, it can cause severe agitation, hypotensive crisis, fever, muscle spasms, convulsions, coma[11]:400. It is not recommended to combine it with fluvoxamine and clozapine. Increases concentrations of SSRI antidepressants[5]. Enhances the effect of adrenomimetics, can enhance the effect of adrenaline, antihistamines with a sedative effect, antithrombotic agents, vitamin K antagonists, atropine, biperiden, isoprenaline, norepinephrine, drugs for general anesthesia, antiparkinsonian drugs, quinidine, ethanol and ethanol-containing drugs, ephedrine. Taking quinidine in combination with clomipramine is not recommended. Clomipramine can weaken the effect of betanidine, clonidine, reserpine (up to the cessation of this effect), weaken the effect of methyldopa [11]: 399-401.
The combination of clomipramine with CNS depressants can lead to dangerous lethargy, as can the combination of clomipramine with opioid analgesics[11]:121. When clomipramine is combined with levodopa, blood pressure may increase; when combined with procainamide, quinidine or thyroid-stimulating drugs, cardiac arrhythmias may occur; when combined with hormonal contraceptives, depression increases; when combined with disulfiram, delirium may occur. Combination with lithium drugs can lead to a decrease in the seizure threshold [11]: 400.
Carbamazepine, phenytoin, nicotine reduce the concentration of clomipramine in the blood plasma; cimetidine, estrogens increase its concentration [11]: 400.
special instructions
Before starting treatment with Anafranil, hypokalemia must be eliminated.
If you have liver disease, the activity of liver enzymes should be monitored during drug therapy.
A good effect is achieved by combining Anafranil with benzodiazepines. During treatment, the dosage of the drug is gradually increased (depending on tolerability), and the benzodiazepine is discontinued. It is advisable that treatment last at least six months.
When treated with Anafranil, you should avoid driving vehicles and operating potentially dangerous mechanisms that require high concentration of attention.
The drug should be discontinued gradually (to avoid adverse reactions).
Use in old age
Anafranil should be used with extreme caution in patients with cardiovascular diseases, mainly arrhythmias, intracardiac conduction disorders (for example, AV block I–III degrees) or cardiovascular failure. In such patients, as well as in elderly patients, regular ECG and monitoring of heart function is recommended.
When taking Anafranil to patients suffering from chronic constipation, special caution is required. It can cause paralytic ileus in both bed rest and elderly patients.
Overdose
An overdose of the drug can have serious consequences, especially in children. Symptoms are similar for all drugs in the tricyclic antidepressant .
Symptoms of an overdose appear within 4 hours after the drug enters the body, their maximum number and severity occurs within a day. The patient should be monitored for 5-6 days.
In case of overdose, the following are observed: drowsiness , anxiety, ataxia , convulsions , increased body temperature, coma , delirium , vomiting, respiratory depression, fever, severe decrease in blood pressure, arrhythmia and tachycardia , shock , stupor, heart failure up to complete cardiac arrest. Possible: anuria , cyanosis or oliguria .
As a therapy, it is necessary to urgently perform gastric lavage. If the patient is conscious, induce vomiting; if not, then intubate the trachea . Call an ambulance, give the victim enterosorbents ( activated carbon ). It is advisable to carry out the operations described above, even if more than 12 hours have passed since the overdose.
The drug does not have a specific antidote hemodialysis and peritoneal dialysis are not used. Therefore, they use modern methods of intensive therapy, eliminate convulsions, and perform artificial ventilation.
Use during pregnancy and lactation
Experience with the use of Anafranil in pregnant women is limited. Due to the presence of isolated reports of a possible connection between treatment with tricyclic antidepressants and the occurrence of malformations in the fetus, the use of the drug during pregnancy is contraindicated. The exception is cases when treatment turns out to be vital for the mother, exceeding the potential risks to the fetus.
If the mother took tricyclic antidepressants such as clomipramine throughout pregnancy, until the onset of birth, the newborns developed a withdrawal syndrome during the first few hours or days of life, expressed in a strong increase or decrease in blood pressure, shortness of breath, increased nervous excitability, drowsiness, intestinal colic , tremors, spastic phenomena or convulsive seizures. To prevent the development of this syndrome, it is recommended to gradually discontinue Anafranil, if possible, at least 7 weeks before the expected onset of labor.
Since clomipramine passes into breast milk, you should either discontinue the drug, gradually reducing the dose, or stop breastfeeding.
Anafranil price, where to buy
The price of Anafranil tablets is approximately 300 rubles for 30 pieces of 25 mg each.
The cost of the solution for injection is 640 rubles for 10 pieces of 2 ml.
- Online pharmacies in RussiaRussia
ZdravCity
- Anafranil tablets p.p.o.
25 mg 30 pcs Novartis Pharma S.р.A RUB 281 order - Anafranil SR tablets p.p.o. prolonged action 75 mg 10 pcs. Novartis Pharma S.р.A
RUB 394 order