Antidepressants are psychotropic drugs that are used in the treatment of depressive disorders. One of the causes of depression is a lack of monoamines, neurotransmitters - serotonin, dopamine, norepinephrine. Medicines affect the level of neurotransmitter hormones. Increasing the level of neurotransmitters improves the patient's mood, increases self-esteem, determination, relieves anxiety, irritability, melancholy, and normalizes appetite and sleep. Antidepressants are classified into:
- Blocking neuronal uptake of monoamines.
- MAO - monoamine oxidase inhibitors.
- Monoamine receptor agonists.
The Yusupov Hospital uses innovative drugs to treat mental disorders, Alzheimer's disease, and dementia. The hospital specializes in the treatment of neurological diseases, uses innovative techniques and medications certified in Russia. Patients are treated on an outpatient basis, in a hospital and in a rehabilitation center. Patients are diagnosed with diseases in the laboratory and diagnostic center of the Yusupov Hospital. Patients are received by doctors of various qualification categories, professors, doctors of science and candidates for doctor of science.
Antidepressants, depending on the clinical effect, are classified into the following groups:
- Drugs with a sedative effect.
- Preparations with a balanced effect.
- Antidepressants-stimulants.
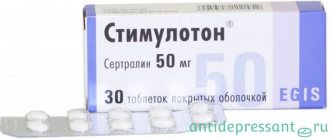
Sertraline belongs to the group of drugs with balanced action.
Compound
The main active ingredient of the drug is sertraline . Its concentration in each tablet is written on the medicine package. The pharmacological properties of Zoloft are due to the effect of sertraline on the body.
In addition to the main active ingredient, the drug contains a number of excipients. They do not affect the body, but affect the absorption of sertraline. Excipients of Zoloft include: calcium, magnesium, sodium, titanium compounds, cellulose and its compounds, hypromellose, polysorbate, polyethylene glycol.
Release form
These are oval (oblong) tablets, on which there are inscriptions about the company that patented this antidepressant and about the dosage of sertraline in the pill.
Thus, Zoloft has “Pfizer” engraved on one side and “ZLT-50” or “ZLT-100” on the other.
The tablets are white in color and have a dividing line; they are packaged in one or two blisters (containing 14 or 28 pieces) in a cardboard package.
Pharmacological properties
Zoloft's mechanism of action is based on selectively blocking the entry of serotonin into neurons after its release. Normally, serotonin is released from neurons in the central nervous system, interacts with other neurons, transmitting certain information to them, and then the molecule of this substance returns back to its neuron.
Serotonin, like other representatives of monoamines, is responsible for the emotional-volitional sphere of human activity. The more it is, the better the mood and the less anxiety. Zoloft, by preventing the entry of serotonin into cells, increases its concentration between cells. The transfer of information between neurons depends on this concentration. At the same time, the synthesis of new serotonin increases in the cells themselves. This is the main mechanism for combating depression, since in this disease the level of monoamines in the brain is significantly reduced.
In addition to serotonin, there are two other important monoamines - dopamine and norepinephrine. Zoloft has little effect on their transmission, due to its selectivity. This explains the lower number of side effects compared to monoamine oxidase inhibitors.
Indirectly through serotonin, Zoloft affects adrenergic transmission. Adrenaline is one of the mediators of the autonomic nervous system. Almost all adverse reactions of Zoloft are associated with its effect on the autonomic system.
Use during pregnancy and lactation
Pregnancy
There are no data from controlled studies of the use of sertraline during pregnancy, so the use of Zoloft during this period is possible only after a thorough analysis of the possible risk to the child and the expected benefit to the mother.
Analysis of a significant amount of data did not lead to the conclusion that the use of sertraline induces birth defects. Animal studies have provided information about the possible effects of sertraline on reproductive function. There is a possibility that this effect is associated with maternal toxicity, which is caused by the pharmacodynamic effects of sertraline on the fetus.
Some newborns whose mothers took sertraline during pregnancy experienced symptoms resembling withdrawal reactions.
Women of reproductive age taking sertraline should use reliable contraceptives.
Lactation
Sertraline and N-desmethylsertraline are found in small amounts in breast milk. In most cases, insignificant concentrations of sertraline were found in the blood plasma of newborns. The exception is one case when 50% of the concentration of sertraline in the mother's blood plasma was found in the blood plasma of the newborn (there was no noticeable effect on the health of the newborn). When prescribing Zoloft during lactation, it is recommended to stop breastfeeding.
Infants whose mothers were treated with Zoloft and other SSRIs or SSRIs during pregnancy experienced complications that required additional hospitalization, tube feeding, and respiratory support. Newborns whose mothers received sertraline during late stages of pregnancy require careful monitoring: such children may develop respiratory distress, cyanosis, seizures, apnea, instability of body temperature, vomiting, feeding difficulties, hypoglycemia, hypo- or hypertonicity, hyperreflexia, twitching muscles, tremors, as well as prolonged crying, increased excitability, drowsiness, lethargy, difficulty falling asleep. The described symptoms may indicate the development of a withdrawal syndrome or may be associated with direct serotonergic effects. Often these complications begin immediately after birth or shortly (less than 24 hours) after birth. It should be borne in mind that in some cases the clinical picture may resemble the symptoms of serotonergic syndrome.
Newborns whose mothers took SSRIs during pregnancy may also have an increased risk of developing persistent pulmonary hypertension of the newborn (PPHN), which accounts for 5 cases per 1000 pregnancies and is one of the causes of morbidity and mortality in newborns. Several recent epidemiological studies have found a possible association between the development of PPHN and the use of SSRIs (including Zoloft).
Fertility
One of two studies in mice showed a decrease in fertility when taking sertraline at a dose of 80 mg per 1 kg of body weight (4 times the maximum recommended dose for humans when calculated mg/m2).
The reported clinical cases indicate that some SSRIs may have a reversible effect on sperm quality.
Indications
Zoloft is a drug that is used quite often in the daily practice of psychiatrists. Its appointment is justified in the following cases:
- Recurrent depressive disorder, prevention and treatment of exacerbations;
- Treatment of a single episode of depression;
- Manic-depressive states, current depressive episode;
- Obsessive thoughts and obsessive actions;
- Treatment and prevention of panic attacks;
- Stress-related disorders;
- Pathological fears.
Zoloft is safer than all tricyclic antidepressants, so it can be used to treat depression of any severity. Unlike other representatives of the group of selective serotonin reuptake inhibitors, Zoloft does not tend to cause an inversion of affect in bipolar affective disorder, that is, the risk of depression turning into mania is minimal. This is the drug of choice for the treatment of this disease.
Zoloft drug interactions
MAO inhibitors. The combined use of sertraline with MAO inhibitors is contraindicated (see SPECIAL INSTRUCTIONS). Other serotonergic drugs (see SPECIAL INSTRUCTIONS). Switching from taking selective serotonin reuptake inhibitors, antidepressants or anti-obsessive drugs (see SPECIAL INSTRUCTIONS). Pimozide. In a study of the combined use of a low dose of pimozide (2 mg once) and sertraline at a dose of 200 mg/day, an increase in the level of pimozide in the blood plasma was noted without any changes in the ECG. Since the mechanism of this interaction has not been established, and given the narrow therapeutic index of pimozide, the simultaneous use of sertraline and pimozide is contraindicated. CNS depressants and alcohol. The combined use of sertraline (200 mg/day) did not potentiate the effect of alcohol, carbamazepine, haloperidol or phenytoin on cognitive and psychomotor performance in healthy individuals; however, the simultaneous use of sertraline and alcohol is not recommended. Lithium. The combined use of sertraline and lithium did not significantly change the pharmacokinetics of lithium, however, tremor increased, which may indicate a pharmacodynamic interaction. With the simultaneous use of sertraline and lithium preparations, which affect serotonergic neuromediation, it is necessary to ensure appropriate control. Phenytoin. Long-term use of sertraline at a dose of 200 mg/day does not lead to clinically significant inhibition of phenytoin metabolism. Despite this, monitoring of phenytoin plasma concentrations during the initial phase of sertraline therapy with appropriate adjustment of the phenytoin dose should be recommended. In addition, concomitant use of phenytoin may cause a decrease in sertraline plasma concentrations. Sumatriptan. There are reports of isolated cases of the development of general weakness, hyperreflexia, impaired coordination, confusion, anxiety and agitation, which were observed after the simultaneous use of sertraline and sumatriptan. If it is necessary to prescribe sertraline and sumatriptan simultaneously, the patient should be under medical supervision. Drugs that bind to blood plasma proteins. Since sertraline binds to plasma proteins, it is necessary to consider the possibility of its interaction with other drugs that also bind to plasma proteins. However, in 3 studies, no significant effect of sertraline on the binding of diazepam, tolbutamide and warfarin to plasma proteins was detected. Warfarin. With simultaneous use of sertraline at a dose of 200 mg/day with warfarin, a slight but statistically significant increase in prothrombin time was observed; the clinical significance of this effect is unknown. In this regard, prothrombin time should be carefully monitored at the beginning of sertraline therapy and after its discontinuation. Interaction with other means. A number of studies have examined the interaction of sertraline with various drugs. The simultaneous use of sertraline at a dose of 200 mg/day with diazepam and tolbutamide led to a slight but statistically significant change in some pharmacokinetic parameters. Cimetidine caused a significant decrease in the clearance of sertraline when used simultaneously. The clinical significance of these changes is unknown. Sertraline did not affect the β-adrenergic blocking activity of atenolol. There were no signs of interaction of sertraline (at a dose of 200 mg/day) with glibenclamide and digoxin. Electroconvulsive therapy . The safety and effectiveness of the combined use of electroconvulsive therapy and sertraline have not been studied in clinical studies. Drugs metabolized with the participation of cytochrome P450 CYP 2D6. Antidepressant drugs have varying abilities to inhibit the activity of the 2D6 isoenzyme of cytochrome P450 CYP 2D6. The clinical significance of this depends on the degree of inhibition and the therapeutic index of the drug prescribed. CYP2D6 substrates with a low therapeutic index include tricyclic antidepressants and class Ic antiarrhythmics such as propafenone, flecainide. In studies of drug interactions with long-term use of sertraline at a dose of 50 mg/day, an increase (on average by 23–37%) in stable plasma levels of desipramine (a marker of CYP 2D6 activity) was detected. Drugs metabolized by other CYP isoenzymes (CYP 3A3/4, CYP 2C9, CYP 2C19, CYP 1A2). CYP 3A3/4: In vivo studies revealed that long-term use of sertraline at a dose of 200 mg/day does not inhibit CYP 3A3/4 dependent 6-β-hydroxylation of endogenous cortisol, or the metabolism of carbamazepine or terfenadine. In addition, long-term use of sertraline at a dose of 50 mg/day did not inhibit CYP 3A3/4, which is involved in the metabolism of alprazolam. The results of this study indicate that sertraline is not a clinically significant inhibitor of CYP3A3/4. CYP 2C9: Chronic use of sertraline 200 mg/day had no effect on plasma concentrations of tolbutamide, phenytoin, and warfarin, indicating that sertraline is not a clinically significant inhibitor of CYP 2C9. CYP 2C19: The use of sertraline at a dose of 200 mg/day did not affect the plasma concentrations of diazepam, indicating that sertraline is not a clinically significant inhibitor of CYP 2C19. CYP 1A2: In vitro studies indicate that sertraline has no or little effect on the activity of CYP 1A2.
Contraindications
There are absolute and relative contraindications to the use of Zoloft. Absolute contraindications include:
- Hypersensitivity to the components of the drug;
- Manic syndrome;
- Use simultaneously with monoamine oxidase inhibitors and some antipsychotics;
- Breast-feeding;
- Childhood.
If the above contraindications are present, Zoloft cannot be prescribed. A study of the effect of the drug on the body of pregnant women has not been conducted, therefore during pregnancy it can be prescribed only if the benefits to the mother outweigh the possible risks to the fetus. The effect of Zoloft on children should be studied in more detail.
Relative contraindications include:
- Organic brain lesions;
- Epilepsy;
- Mental retardation of any severity;
- Decompensated diseases of internal organs;
- Cachexia or low body mass index.
If there is at least one relative contraindication, the risk of prescribing the drug should be assessed by a doctor. Using the product in this case is possible, but not recommended if there is a safer alternative.
Practical scientific research on the effectiveness of Zoloft
Modern scientists in the 90s-2000s conducted a number of experiments studying the effect of the drug.
In America, scientists examined a group of volunteers with chronic depression who took medication for 3-4 months. As a result, doctors noted the absence of new exacerbations of the disease in 74% of patients.
New studies have also been conducted to study the effectiveness of Zoloft therapy for seasonal depression (they are not officially an indication for its use).
In this experiment, patients took an antidepressant for 8 weeks. Analysis of the results showed a statistically significant decrease in clinical manifestations in the control group, and, therefore, a positive effect of the drug.
In 1995 , doctors found that Zoloft can also be used in the treatment of atypical depression. Experiments in this area have confirmed its effectiveness - 97% of patients noted significant relief after an eight-week course of taking this antidepressant.
In 2016-2018 , data appeared on studies by American scientists who confirmed the relevance of Zoloft treatment for patients with dysthymia. Experiments have shown that treatment with Zoloft along with the use of psychotherapy is more effective than using it alone.
There are scientific works that reveal the positive effects of Zoloft on postpartum depression, childhood post-traumatic diseases, mood disorders due to alcoholism and drug addiction.
Side effects
The most common side effect of Zoloft is nausea, which occurs after taking the pills. In addition, men may experience a lack of erection and a significant decrease in libido. It has been proven that these reactions are associated specifically with taking Zoloft. However, with regular use of the drug, these side effects disappear on their own and do not require treatment.
Other reactions are somewhat less common. Dizziness, headache, insomnia or drowsiness may occur. Many patients experience tremors in the arms and legs, which disappear with continued use of Zoloft. Another common side effect is a decrease or complete absence of appetite. The manufacturer's official instructions indicate that Zoloft is an anorexigenic drug.
Among other things, Zoloft can affect the autonomic nervous system. This causes a number of common side symptoms: diarrhea, dry mouth, dry eyes, increased heart rate, increased blood pressure. The listed conditions do not disappear on their own; they require specific treatment or a change in medication.
Zoloft inhibits the uptake of serotonin not only by neurons in the brain, but also by blood platelets, so platelets in the blood may decrease during treatment. This increases the risk of bleeding, especially nosebleeds, from small wounds, and petechial rashes on the skin. In women, taking Zoloft may be accompanied by prolonged, heavy menstruation, requiring specific treatment.
Zoloft affects metabolic processes in the liver. With prolonged use, it is possible to enlarge the liver, increase transaminases in the blood test, and in rare cases, a bitter taste in the mouth and visible jaundice.
As with any other medication, an allergic reaction may occur during treatment with Zoloft. In most patients with hypersensitivity, it manifests as urticaria. In rare cases, angioedema may occur. If these complications develop, it is necessary to urgently stop taking the drug and begin the administration of antihistamines and steroids.
Zoloft®
Sertraline should not be co-administered with MAOIs, within 14 days before starting an IMAO and for 14 days after their discontinuation.
Blood concentrations of tricyclic antidepressants should be monitored to assess the need for dose adjustment.
When using sertraline and golbutamide simultaneously, it is necessary to monitor blood glucose levels (see section “Interaction with other drugs”).
Serotonin syndrome
Cases of the development of serotonin syndrome (SS) and neuroleptic malignant syndrome (NMS) have been described with the use of SSRIs. The risk of these complications increases with simultaneous use of SSRIs with other serotonergic drugs (including trintanes and fentanyl and their analogues, tramadol, dexomstorphan, tapentadol, meperedine, methadone, pentazocine), as well as drugs that affect the metabolism of serotonin (including monoamine oxidase inhibitors ), antipsychotics and other dopamine receptor antagonists. Manifestations of SS may include changes in mental status (in particular, agitation, hallucinations, coma), autonomic lability (tachycardia, blood pressure fluctuations, hyperthermia), changes in neuromuscular transmission (hyperreflexia, impaired motor coordination) and/or gastrointestinal disorders (nausea, vomiting and diarrhea). Some manifestations of SS, including hyperthermia, muscle rigidity, autonomic lability with possible rapid fluctuations in vital signs, as well as changes in mental status, may resemble symptoms that develop in NMS. It is necessary to monitor patients for the development of clinical manifestations of SS and PVD.
Prolongation of the OTc interval or arrhythmia ventricular tachysystolic tina “pirouette” (torsade de pointes)
During post-marketing use of sertraline, cases of prolongation of the QTc interval on the ECG and the development of ventricular tachysystolic arrhythmia of the torsades de pointes type have been reported. Most cases were observed in patients with risk factors for developing such conditions. Therefore, caution should be exercised when using sertraline in patients with risk factors for prolongation of the QTc interval on the ECG or the development of torsade dc pointes.
Switching from other SSRIs, antidepressants or anti-obsessive medications
The required interval between stopping one SSRI and starting another similar drug has not been established. Caution should be exercised when switching to sertraline from other SSRIs, antidepressants or anti-obsessive medications, especially from long-acting medications such as fluoxetine.
When replacing one neuronal serotonin uptake inhibitor with another, there is no need for a washout period. However, caution is required when changing the course of treatment.
Other serotonergic drugs, such as tryptophan, (fenfluramine and 5-HT agonists
The simultaneous use of sertraline with other drugs with a pronounced effect on neurotransmitter transmission (such as tryptophan, fenfluramine, 5-HT agonists or herbal medicines, St. John's wort) should be used with caution and, if possible, avoided, given the potential pharmacodynamic interaction.
Suicidal behavior
Depression is associated with an increased risk of suicidal ideation, self-harm, and suicide. This risk persists until stable remission. Given that improvement in the patient's condition may not occur in the first few weeks of therapy or longer, patients should be closely monitored until such improvement occurs. It is also common for the risk of suicide to increase during the early stages of recovery.
Other medical conditions for which sertraline may be prescribed may also be associated with an increased risk of suicidal events. In addition, these diseases may accompany major depressive disorder. In this regard, the same precautions should be taken as in the treatment of major depressive disorder.
Patients with a history of suicidal tendencies or patients prone to suicidal ideation before starting therapy have a higher risk of suicidal thoughts or suicide attempts. Such patients should also be under close medical supervision during therapy.
All patients, especially those at risk, receiving sertraline therapy should be carefully monitored to detect the development or worsening of symptoms of suicidal behavior. Patients, their relatives and guardians should be warned of the need to monitor the condition for the emergence or worsening of depression, the emergence of suicidal thoughts or behavior, as well as for any changes in behavior, especially at the beginning of therapy and with any change in the dose of the drug. The risk of suicide attempts should also be kept in mind, especially in patients with depression. In this regard, in order to reduce the risk of overdose, it is necessary to take the minimum dose of the drug that provides a sufficient therapeutic effect.
Patients with depression and other mental disorders are at risk of suicidal behavior. These diseases themselves are strong predisposing factors for such behavior. In children, adolescents and young adults (ages 18–24 years) with depression or other mental disorders, antidepressants (SSRIs and others) have been found to increase the risk of suicidal ideation and behavior compared with placebo. Therefore, when using sertraline or any other antidepressants in children, adolescents and young adults (under 24 years of age), the risk of suicide should be weighed against the benefits of their use. In addition, there was no increase in the risk of suicidal behavior in adult patients over 24 years of age, and a decrease in this risk was noted in patients aged 65 years and older.
Use in children and adolescents under 18 years of age
Sertraline should not be used to treat children and adolescents under the age of 18 years, with the exception of patients with OCD aged 6-17 years. Suicidality (suicide attempts or suicidal thoughts) and hostility (primarily aggressiveness, oppositional behavior and anger) were observed more often in patients receiving antidepressant therapy than in patients receiving placebo. If a decision is made to proceed with therapy based on the clinical assessment of the patient, the patient should be carefully monitored for symptoms of suicidal behavior. In addition, it should be borne in mind that data on the effect of the drug on growth, puberty and cognitive and behavioral development of the child are limited. During long-term therapy of pediatric patients, clinicians should monitor for abnormal developmental abnormalities.
Withdrawal syndrome
When stopping a drug, withdrawal symptoms often occur, especially if the drug is stopped abruptly. Withdrawal symptoms were observed in 23% of patients who stopped taking sertraline and in 12% of patients who continued taking the drug. The risk of these symptoms depends on several factors, including the duration of therapy and dosage, and the rate of dose reduction. The most common reactions are dizziness, sensory disturbances (including paresthesia), sleep disturbances (including insomnia and deep sleep), agitation or anxiety, nausea and/or vomiting, tremor and headache. These symptoms are usually mild to moderate in severity; however, in some cases they can be severe. Typically, these symptoms occur during the first few days of discontinuation of therapy, but there are very rare reports of the development of such symptoms in patients who inadvertently missed a dose. Typically these symptoms do not get worse and resolve within two weeks, except in some cases where they may last longer (2-3 months or more). In this regard, it is recommended to discontinue the drug gradually, reducing the dose over several weeks or months, depending on the patient's condition.
Akathisia/nsychomotor agitation
The use of sertraline may be associated with the development of akathisia, characterized by a subjective feeling of discomfort or restlessness and a need to move, accompanied by an inability to sit or stand still. Most often, such symptoms are observed in the first weeks of treatment. Increasing the dose in these patients may be harmful.
Liver dysfunction
If it is necessary to use sertraline in patients with impaired liver function, consider reducing the dose of the drug or the frequency of administration. Sertraline should not be taken in patients with severe hepatic impairment.
Renal dysfunction
It was found that. As expected, given the insignificant renal excretion of sertraline, no dose adjustment is required depending on the severity of renal failure.
Electroconvulsive therapy
The possible success or risk of this combination treatment has not been studied (clinical data are not available).
Convulsions
There is no experience with the use of sertraline in patients with convulsive syndrome, so its use should be avoided in patients with unstable epilepsy, and patients with controlled epilepsy should be carefully monitored during treatment. If seizures occur, the drug should be discontinued.
Activation of mania/hypomania
During clinical studies prior to the marketing of sertraline, hypomania and mania were observed in approximately 0.4% of patients receiving sertraline. Cases of activation of mania/hypomania have also been described in a small proportion of patients with manic-depressive psychosis who received other anti-depressive or anti-obsessive drugs. Sertraline should be used with caution in patients with a history of mania or hypomania. Close medical supervision is necessary and sertraline should be discontinued if the patient exhibits any signs of mania.
Schizophrenia
Patients with schizophrenia may experience exacerbation of psychotic symptoms.
Pathological bleeding/hemorrhage
There are reports of the development of bleeding or hemorrhage from ecchymosis and purpura or life-threatening bleeding/hemorrhage) during the use of SSRIs. Caution should be exercised when prescribing SSRIs in combination with drugs that have an established ability to affect platelet function (for example, atypical antipsychotics and phenothiazines, most tricyclic antidepressants, acetylsalicylic acid and non-steroidal anti-inflammatory drugs), as well as in patients with a history of hemorrhagic diseases.
In addition, when using sertraline with indirect anticoagulants, it is recommended to monitor the prothrombin time at the beginning of treatment with sertraline and after its discontinuation.
Hyponatremia
Transient hyponatremia occurs more often in older patients, in patients with dehydration, or when taking diuretics. This side effect is associated with the syndrome of inappropriate secretion of antidiuretic hormone. Cases of decreased plasma sodium concentrations below 110 mmol/L have been reported. If automatic hyonatremia develops, sertraline should be discontinued and adequate therapy aimed at correcting the concentration of sodium in the blood should be prescribed. Signs and symptoms of hyponatremia include headache, difficulty concentrating, memory loss, weakness and unsteadiness, which can lead to falls. In more severe cases, hallucinations, fainting, seizures, coma, respiratory arrest, and death may occur.
Due to the fact that there is a clear relationship between the development of depression and OCD, depression and panic disorders, depression and PTSD. depression and social phobia, when treating patients with OCD, panic disorders, PTSD and social phobia, the same precautions should be taken as when treating depression.
Fractures
Based on epidemiological studies, it has been established that the use of serotonin reuptake inhibitors, including sertraline, increases the risk of fractures. The mechanism leading to the increased risk is not completely clear.
Elderly patients
The profile of adverse reactions in elderly and young patients is not different. In old age, the drug should be used with caution due to the increased risk of developing hyponatremia.
Diabetes mellitus/impaired blood glucose control When using SSRIs, including Zoloft®, cases of exacerbation of diabetes mellitus and/or impaired glucose control (hyperglycemia and hypoglycemia) have been reported in patients with or without diabetes mellitus. In this regard, glucose levels should be monitored. Particular attention is required in patients with diabetes mellitus, as they may require dose adjustment of oral hypoglycemic agents and/or insulin.
Angle-closure glaucoma
SSRIs, including sertraline, affect pupil size, leading to mydriasis. In this case, a narrowing of the angle of the eye is observed, which leads to an increase in intraocular pressure and the development of closed-angle glaucoma, especially in patients with a predisposition. The drug should be used with caution in patients with angle-closure glaucoma or a history of glaucoma.
Laboratory methods
False-positive urine immunoassays for benzodiazepines have been reported in patients taking sertraline. This is due to the low specificity of screening tests. False-positive results may also occur for several days after discontinuation of sertraline therapy. Additional tests, such as gas chromatography and mass spectrometry, can help distinguish sertraline from benzodiazepines.
Grapefruit juice
The simultaneous use of sertraline and grapefruit juice is not recommended.
Overdose
During monotherapy, no overdose of Zoloft was observed. Symptoms of overdose occurred when Zoloft was combined with other drugs, primarily antidepressants from other groups, as well as when taken together with alcohol. You should learn more about the interaction of Zoloft and alcohol.
Symptoms of overdose are similar to adverse reactions, but may be more severe. Most often, nausea and vomiting occur, so the body tries to get rid of excess drugs. Some patients may experience diarrhea. Tachycardia and increased blood pressure, pathological drowsiness often occur. Impaired consciousness may develop, leading to coma.
In such patients, it is necessary to monitor the electrocardiogram, since an overdose of Zoloft can lead to ventricular tachycardia, turning into ventricular fibrillation. The latter is the cause of cardiac arrest and clinical death.
There is no specific treatment for Zoloft overdose. If symptoms of overdose occur, it is necessary to take sorbents orally and call emergency medical help. Treatment consists of detoxification therapy and maintaining vital body functions.
Zoloft and alcohol
absolutely unacceptable combination, fraught with the development of coma and death of patients.
The simultaneous use of these substances causes severe poisoning and intoxication of the body, occurring with nausea, vomiting, dizziness, confusion, diarrhea, excessive agitation or stupor, the occurrence of pathologically enhanced reflexes, and convulsions.
Instructions for the drug
The daily dose of Zoloft should not be divided into several doses. The drug is taken once a day at any time. Food intake does not affect the absorption and distribution of Zoloft. The dose of the drug depends on the specific disease and the duration of the medication. There is a starting dose, which is prescribed at the very beginning of treatment, and an average therapeutic dose, which has the best therapeutic effect. There is also a maximum dosage - one that cannot be exceeded even if the therapeutic effect is not achieved.
When treating depression and obsessions, you should start with 50 mg per day. After a week, you can double the dose to 100 mg per day. Thus, you can increase the dose by 50 mg per week until the desired effect is achieved or a maximum dosage of 200 mg per day.
To treat any manifestations of anxiety-phobic disorder, you should start with half the dose of 25 mg per day. In a week it can be doubled. As a rule, this is enough to achieve a therapeutic effect. If the desired result is not achieved, the dose can be increased by another 25 mg.
The drug begins to act no earlier than after a week of regular use. In this case, the desired result will appear no earlier than after 14 days. Zoloft should be taken for at least three months.
Abruptly stopping treatment is not recommended due to the possibility of developing withdrawal syndrome.
Dosage when taking Zoloft
As with all medications, follow your prescription instructions. Zoloft is taken orally once a day, in the morning or evening. It is available in two formulations.
- Tablets are available in dosages of 25mg, 50mg, and 100mg. Must be taken with water or other liquids.
- The oral solution should be measured using the provided dropper and poured into water, lemon soda, lemonade or orange juice, stirred, and then swallowed completely.
The optimal dosage varies depending on the treatment conditions. If you are over 65 or have certain health problems, your doctor may recommend a lower dosage.
Your doctor may gradually adjust your daily dosage until you find the lowest dosage at which you experience the greatest improvement in symptoms without side effects.
Abruptly stopping Zoloft can have serious consequences, including irritability, mood changes, anxiety, difficulty sleeping, headache, sweating, nausea, dizziness, etc.
Analogs
There are a number of drugs that have the same active ingredient as Zoloft. They are also generics, but are produced by other companies. These analogues of Zoloft are:
- Asentra;
- Default;
- Seralin;
- Emoton;
- Thorin;
- Aleval;
- Misol;
- Seranta;
- Surlift;
- Stimuloton.
All of the listed medications are similar in pharmacological properties and the principle of action on the body, but differ in delivery methods, half-life, contraindications and side effects. Detailed information is provided in the instructions for each of the listed drugs. When changing one analogue to another, you must consult your doctor.
Price
The cost of the medicine depends on its dosage, the number of blisters and the manufacturer.
So tablets (14 units) from Pfizer Italy can be bought for 512-630 rubles , and the price for two blisters in a box (28 units) from the same company is 1035-1070 rubles .
Pack of 28 pcs. 100 mg each will cost at least 1300 rubles .
to buy Zoloft without a prescription in Russia, since the drug belongs to list B, that is, potent drugs used strictly as prescribed by a doctor.
Reviews
Andrey Z .: “I took Zoloft because of an anxiety disorder that I began to suffer from relatively recently. The most difficult days were the three days of treatment, during which I had terrible heart palpitations and my blood pressure constantly jumped. Then all the side effects went away. I took 50 mg for one month, then gradually reduced the dose. The entire treatment lasted two months - even less than the doctor expected. The anxiety symptoms went away and never came back.”
Alexandra M .: “Several years ago I was treated for both severe depression and obsessive-compulsive disorder. I took Zoloft for six months, 200 mg every day, and after about 3 months I noticed a significant improvement in my condition. Before this, the effect was almost invisible. Six months later, I switched to taking 100 mg per day, and took it in this regimen for a year. After that, the dosage was further reduced to 50 mg, and so I took it for two and a half years. In total, there was four years of treatment with relapse prevention. After this, Zoloft was gradually discontinued, but the symptoms did not return. Among the side effects, for four years I was tormented by pain in the liver, a bitter taste in the mouth and decreased libido. After discontinuation, all symptoms disappeared.”
Review from a psychiatrist: “Zoloft is an effective remedy for combating depression, obsessions and anxiety. Our American colleagues prescribe it to most patients with the listed disorders. For psychotherapists, this drug is the number one remedy. There are a lot of antidepressants similar to Zoloft, so you can choose a different drug when treating depression. But in the fight against obsessions, Zoloft has no equal. In domestic psychiatry, Zoloft is used much less frequently than abroad. We give preference to more affordable analogues with the same active ingredient.”
Content
Who is Zoloft for?
The antidepressant is used to treat the following problems, some of which commonly occur along with attention deficit hyperactivity disorder:
- depressive conditions and to prevent depressive relapses in people with depressive episodes,
- obsessive-compulsive disorders in adults and children over 6 years of age,
- panic attacks
- from some manifestations of anxiety (social phobia, post-traumatic stress disorder)
special instructions
The drug is prescribed with caution in combination with certain medications; it is prohibited to combine it with monoamine oxidase inhibitors. Other points from the special instructions section of the instructions for use read:
- During treatment with the drug, the development of serotonin syndrome and a malignant neuroleptic condition is possible; the risk increases when combined with triptans, antipyrine and antipsychotics. Signs of these abnormalities include tachycardia, pressure fluctuations, hyperthermia, and hyperreflexia.
- Hypomania and mania develop in 0.4% of patients.
- With liver cirrhosis, the elimination period of the drug increases. Zoloft should be used with caution and with adjustment of the dosage interval for liver diseases. In case of kidney failure, the dosage and regimen do not change.
- Transient hyponatremia may occur during sertraline therapy. When it appears, treatment is canceled and adequate therapy is prescribed. Signs of hyponatremia include headache, impaired concentration and memory, fainting, hallucinations, and weakness.
- It is not recommended to drive cars or dangerous machinery while taking the medicine, as the speed of psychomotor reactions decreases.