Momat Rino, 50 mcg/dose, 120 doses, metered-dose nasal spray, 1 pc.
As with any long-term treatment, patients using Momat Rhino nasal spray for several months or longer should be periodically examined by a doctor for possible changes in the nasal mucosa and the possible development of systemic side effects. If a local fungal infection of the nose or throat develops, it may be necessary to discontinue treatment with Momat Rhino nasal spray and undergo special treatment. Irritation of the nasal and pharyngeal mucosa that persists for a long time may also serve as a reason to discontinue treatment with this drug.
Patients who switch to treatment with Momat Rino nasal spray after long-term treatment with systemic GCS require special attention. Withdrawal of systemic corticosteroids in such patients can lead to insufficient adrenal function, the subsequent recovery of which may take up to several months. If signs of adrenal insufficiency appear, systemic corticosteroids should be resumed and other necessary measures taken.
During the transition from treatment with systemic corticosteroids to treatment with Momat Rhino nasal spray, some patients may experience initial withdrawal symptoms of systemic corticosteroids (for example, joint and/or muscle pain, fatigue and depression), despite a decrease in the severity of symptoms associated with the lesion. nasal mucosa; such patients must be specifically convinced of the advisability of continuing treatment with Momat Rino nasal spray.
The transition from systemic to local GCS can also cause pre-existing allergic diseases, such as allergic conjunctivitis and eczema, that were masked by systemic GCS therapy.
Patients undergoing treatment with corticosteroids have a potentially reduced immune reactivity and should be warned about their increased risk of infection in case of contact with patients with certain infectious diseases (for example, chicken pox, measles), as well as the need for medical advice if such contact occurs .
If signs of a significant bacterial infection appear (for example, fever, persistent and sharp pain on one side of the face or toothache, swelling in the orbital or periorbital area), immediate medical consultation is required.
The effectiveness and safety of mometasone have not been studied in the treatment of unilateral, irregularly shaped polyps, bleeding polyps, polyps associated with cystic fibrosis, and polyps that completely occlude the nasal cavity. Unilateral polyps that are irregular in shape or bleeding should be further examined.
With long-term use of nasal corticosteroids in high doses, systemic side effects may develop. The likelihood of developing these effects is much less than with the use of systemic corticosteroids and may vary in individual patients, as well as between different corticosteroids.
Potential systemic effects include Cushing's syndrome, Cushingoid features, adrenal suppression, growth retardation in children and adolescents, cataracts, glaucoma, and less commonly, a number of psychological or behavioral effects including psychomotor hyperactivity, sleep disturbance, anxiety, depression, or aggression (especially in children).
It is recommended to regularly monitor the growth of children receiving long-term therapy with mometasone. If growth slows, therapy should be reconsidered in order to reduce the dose of mometasone to the minimum effective dose to control the symptoms of the disease. In addition, the patient should be referred to a pediatrician for consultation.
Treatment with GCS in higher doses than recommended may lead to clinically significant suppression of adrenal function. If it is known that high doses of corticosteroids are used, it is necessary to consider the possibility of additional use of systemic corticosteroids during periods of stress or planned surgical intervention.
Impact on the ability to drive vehicles and engage in other activities. No data.
Release forms and composition of the drug
Momat Rino is produced in the form of a nasal spray. The liquid inside the bottle has a thick, translucent white texture.
The therapeutic effect of the drug is ensured by the following components:
| List of spray components | Purpose of the component in the medication |
| Main active ingredient | |
| Momentazone furoate monohydrate | It is a glucocorticosteroid, has anti-inflammatory, antipruritic and antihistamine properties, and also promotes vasoconstriction. |
| Additional components (provide the spray with the desired texture and enhance the healing effect of the main element) | |
| Avicel RC-591 | Is a stabilizer |
| Glycerol | Used as a binder |
| Citric acid monohydrate | The element helps strengthen the immune system, has antiseptic properties, and also attracts and removes excess fluid from the body. |
| Sodium citrate dihydrate | The substance is a preservative and also enhances the effectiveness of the main component. |
| Polysorbate 80 | It is used as a binding element and is also a stabilizer. |
| Benzalkonium chloride | It is a strong antiseptic and helps destroy viral and fungal infections. |
| Water for injections | Water gives the medication the desired concentration and consistency |
The product is placed in plastic spray bottles with a dispenser cap. One bottle can contain 60 or 120 doses (1 dose is 50 mcg of the main substance, this amount of product is injected into the nasal cavity after one click on the dispenser).
Pharmacological properties
Pharmacodynamics
Mometasone is a synthetic GCS (glucocorticosteroid) intended for topical use. In doses that do not cause systemic effects, it has antiallergic and anti-inflammatory effects. Mometasone inhibits the release of inflammatory mediators by increasing the production of lipomodulin, which is an inhibitor of phospholipase A. Phospholipase A, in turn, reduces the release of arachidonic acid, and therefore reduces the synthesis of its metabolic products - prostaglandins and cyclic endoperoxides.
Mometasone prevents the accumulation of neutrophils near the walls of blood vessels, which reduces the production of lymphokines and the amount of inflammatory exudate, slows down the migration of macrophages, and also reduces the processes of infiltration and granulation. Momat Rino reduces inflammation by reducing the formation of a chemotaxis substance (affects the later stages of allergies), and slows down the development of immediate allergic reactions (this phenomenon is associated with inhibition of the production of arachidonic acid metabolites and a decrease in the release of inflammatory mediators from mast cells).
Pharmacokinetics
When using Momat Rino intranasally, the bioavailability of mometasone furoate for systemic absorption is less than 1%. The suspension is very poorly absorbed in the digestive tract. A small amount of absorbed mometasone, which theoretically can enter the digestive system after nasal inhalation, undergoes a first-pass effect through the liver and is actively metabolized even before being excreted in bile or urine.
At what age can the drug be used?
The spray has age restrictions, since the active substance is hormonal.
Depending on the purpose of use, the drug has the following age limits:
- from 2 years - when eliminating an allergic reaction, both seasonal and year-round;
- from 12 years of age – when blocking the development of the inflammatory process in the nasal mucosa;
- from 12 years of age – prevention of the development of an allergic reaction;
- from 18 years of age – for the treatment of polyps in the nasal cavity.
Also, special control during the period of use of the spray is required for patients over 65 years of age. These restrictions are caused by the presence of chronic diseases that are present by the time a person reaches a given age.
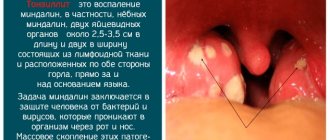
Indications for use
- year-round and seasonal allergic rhinitis (in children over 2 years old, adolescents and adults);
- acute rhinosinusitis with moderate or mild symptoms and no signs of severe bacterial infection (in adults and adolescents 12 years of age and older);
- nasal polyposis, which is accompanied by impaired sense of smell and nasal breathing (in patients over 18 years of age);
- exacerbation of chronic sinusitis and acute sinusitis (as an adjuvant in combination with antibiotics in adolescents over 12 years of age and adults, including elderly patients);
- prevention of moderate and severe seasonal allergic rhinitis 2–4 weeks before the expected start of the dusting season (in adolescents from 12 years of age and adults).
Patient reviews
Maria Viktorovna, 57 years old
I suffer from an allergy to house dust. We do wet cleaning in the apartment every day, but still there are often exacerbations, an allergic runny nose begins. The doctor advised me to use this spray. After a course of therapy, the runny nose went away.
Nikolay, 40 years old
I work at a construction site. There is a lot of dust, construction debris, sand, and concrete at the construction site. As a result of exposure to unfavorable environmental factors, an allergy appeared, a runny nose, cough and lacrimation began. I consulted an allergist. The doctor prescribed inhalations with this medicine. I used it for several days and the allergy went away.
Stepan Gavrilovich, 61 years old
On weekends we went to the dacha and spent the whole day working in the garden. When we arrived home, I noticed that I had an allergic runny nose to flowering plants. I consulted an allergist who prescribed this medication. I inhaled this drug. After a few days of treatment, the allergic reaction went away.
Overdose
Long-term therapy with high doses of Momat Rino, as well as the simultaneous use of several corticosteroids, can lead to inhibition of the function of the hypothalamic-pituitary-adrenal system.
Since the bioavailability of Momat Reno is low (less than 1%), the likelihood that in the event of an intentional or accidental overdose, any immediate measures to eliminate the consequences of an overdose (other than observation and possible subsequent resumption of the drug in regular doses) will be required is very small.
Analogs
If it is necessary to replace the spray, experts recommend the following analogues:
| Spray name | Active ingredient and features of use | Price and form of sale |
| Galazolin Allergo | The spray contains a similar active ingredient. The drug is approved for use after reaching 18 years of age. The course of treatment is no more than 3 months. The dosage is similar to Momat Rhino. | 350 rub. Sold with a prescription |
| Desrinitis | The spray contains a similar active substance. Age restrictions, as well as the dosage of the product are similar to Momat Rino. | 400 rub. Need a prescription upon purchase |
| Nasonex | 500 rub. Sold by prescription | |
| Nosephrine | 500 rub. Available with prescription |
When choosing analogues, it is necessary to take into account the reason for replacement. That is, if the composition does not suit you, then you need to study the components of the drug. If side effects occur, it is necessary to study their presence in the new medication. It is also important to familiarize yourself with contraindications and age restrictions.
Terms, conditions of sale and storage
It is recommended to store Momat Rino in cardboard packaging (it will protect the bottle from sunlight) at a temperature of 15-25 degrees above zero. Freezing the product is strictly prohibited. If the spray is accidentally exposed to frost for a long time, it should be replaced with a new one. If the rules described above are followed, the shelf life of the spray will be 2 years.
Important. The spray should be kept closed and out of reach of children. You can purchase the product only with a prescription.