Sotalex is an antiarrhythmic drug that combines high efficiency and safety acceptable to doctors and patients. A distinctive feature of the drug is its dose-dependent mechanism of action. In a relatively low dosage, the active component of the drug has a β-adrenergic blocking effect, and in a large dosage it acts as an antagonist of potassium channels located on the membrane of cardiac muscle cells (cardiomyocytes).
Sotalex is classified as a non-selective β-blocker (abbreviated as BAB). Previously, the lack of selectivity of action was considered rather a disadvantage of this group of medications.
This was associated with the peculiarities of the localization of β-adrenergic receptors (in the kidneys, bronchi, nervous system, reproductive organs). Accordingly, the risk of adverse reactions while taking non-selective beta blockers increases.
But recently it was found that in addition to β1-adrenergic receptors, β2 and β3 adrenergic receptors are located in the heart muscle, which perform a number of vital functions. This influences the strength and frequency of myocardial contractions, preventing left ventricular hypertrophy, which often occurs in patients with persistent arterial hypertension.
In addition, in patients suffering from long-term damage to the cardiovascular system, the number of β1-adrenergic receptors in the myocardium is significantly reduced. This is reflected in the effectiveness of selective beta blockers. And for certain diagnoses, the lack of selectivity is an advantage. Sotalex (unlike group analogues) is prescribed for arrhythmias of various etiologies and pathogenesis.
Brief information about the manufacturer
The drug Sotalex (Latin non-proprietary international name - Sotalex) is produced by the pharmaceutical company Bristol-Myers Squibb, headquartered in New York, USA. Since the merger of two separate American companies, Bristol-Myers and Squibb, in 1989, the corporation has been actively developing drugs intended for use in oncological and rheumatological practice, prescribed for the treatment of a variety of diseases, including life-threatening ones.
Bristol-Myers Squibb specialists do not ignore common pathologies of the cardiovascular system. The company's branches are located all over the world, including in Russia, which allows it to reach the maximum number of patients.
Instructions for use
Clinical trials and practical experience of use have proven that Sotalex is a good drug that effectively “works” both to maintain the normal rhythm of myocardial contractions and to prevent such disorders after implantation of a pacemaker and other surgical interventions. Before using the medication, you should read the instructions for use.
Dosage form
The drug is produced in the form of tablets containing the active ingredient 160 mg and 80 mg.
Description and components
The active ingredient of the drug is sotalol hydrochloride in quantities of 160 and 80 mg. Additionally, the composition includes cellulose, lactose and magnesium compounds. Tablets are round in shape, convex on both sides, with a longitudinal dividing strip applied.
Pharmacological group
Sotalex is classified as a class III antiarrhythmic drug; according to its principle of action, it is a non-selective β-adrenergic receptor blocker.
Pharmacodynamics
Sotalol, which is part of the drug, indiscriminately blocks β-adrenergic receptors located in myocardial cells and other tissues of the body. As a result, the drug lengthens the action potential in the atria and ventricles. The severity of the clinical effect correlates with the dose.
Sotalol:
- reduces heart rate;
- regulates the contractility of the heart muscle;
- reduces atrioventricular conduction;
- shortens the refractory period (the time lasting from the moment the action potential occurs, followed by a decrease in excitation and restoration of excitability).
Sotalex increases the QT interval, which, if the treatment is incorrectly selected, can cause torsades de pointes (in the medical literature, this syndrome is often called torsades de pointes arrhythmia).
Pharmacokinetics
In accordance with the data given in the description of the drug:
- Adsorption . The active substance of the drug is quickly and almost completely (more than 90%) absorbed from the digestive tract. To achieve maximum concentration, 2.5 - 4 hours is enough. The equilibrium level of sotalol is observed several days after the start of treatment. Food intake negatively affects pharmacokinetic properties.
- Distribution . The drug substance does not bind to proteins, so the level of distribution is dose-dependent. Sotalol has hydrophilic properties, practically does not pass through the blood-brain barrier, and no more than 10% is found in the cerebrospinal fluid.
- Biotransformation . The active ingredient of the drug for the most part does not undergo chemical transformations in the liver.
- Removal . The drug is predominantly (80-90%) excreted in urine, the rest in feces. The half-life ranges from 10 to 20 hours. On average, the effect of the medication lasts up to a day.
Pharmacokinetic parameters are affected by the patient’s age and concomitant renal pathologies. Thus, in elderly patients, a tendency of sotalol to accumulate in the tissues of the body was noted. In chronic renal failure, the half-life of the drug can increase to 2 days.
Information about the main active ingredient
The active component sotalol in its chemical structure is a mixture of two optical isomers. Levorotatory has a pronounced β-adrenergic blocking effect. At a dosage of 0.16 g per day and above, K+ channels are blocked.
The therapeutic properties of sotalol were discovered in 1970, but drugs containing this substance have been used in official medicine since 1993.
List of indications for taking medication
Sotalex is prescribed for the following diseases and syndromes:
- prevention of potentially fatal ventricular tachyarrhythmias;
- symptomatic correction of paroxysmal ventricular tachyarrhythmias;
- supraventricular arrhythmias;
- prevention of paroxysmal tachycardia, atrial fibrillation, paroxysmal recurrent tachycardia;
- paroxysmal tachycardia AV-Re-entry;
- ventricular arrhythmias of a constant or paroxysmal nature, against the background of electrical stimulation of the myocardium;
- arrhythmia arising in connection with pathologies of the conduction system of the heart;
- symptomatic treatment of tachycardia that occurs due to thyroid diseases;
- angina pectoris;
- hypertension in combination with tachycardia;
- hypertrophic cardiomyopathy;
- mitral valve prolapse;
- relief of arrhythmia due to myocardial infarction;
- paroxysmal supraventricular tachycardia after surgery.
Sotalex is also used to restore the physiological functioning of the myocardium after atrial fibrillation or fibrillation. Taking the medication is advisable for almost all tachyarrhythmias of supraventricular origin and heart rhythm disorders caused by pathological sensitivity or increased levels of catecholamines.
List of contraindications and restrictions
The list of absolute contraindications to the use of Sotalex includes:
- individual intolerance to the main and auxiliary components of the drug;
- age restrictions (taking medication by patients under 18 years of age is potentially dangerous);
- sick sinus syndrome;
- sinoatrioventricular block (in the absence of a pacemaker);
- prolongation of the QT interval, regardless of etiology (including medication);
- decompensated form of chronic heart failure;
- some forms of angina;
- pulmonary hypertension;
- acute course of myocardial infarction;
- cardiogenic shock;
- surgery performed under anesthesia, which depresses cardiac activity;
- active course of pheochromocytoma;
- hypotension;
- severe kidney damage;
- Raynaud's syndrome and other pathologies of peripheral circulation;
- bronchospasm, bronchial asthma, obstructive lesions of the respiratory tract;
- pregnancy and lactation;
- metabolic acidosis.
Relative contraindications to the use of the drug are mild to moderate renal failure, decreased levels of K+ and Mg2+, and a decompensated form of diabetes mellitus (especially with a history of hypokalemia).
Features of administration and dosage
The initiation of therapy and subsequent dosage adjustments are carried out only under the supervision of a physician with mandatory checking of the ECG (pay attention to the QT interval), parameters of the urinary system, and the content of basic electrolytes. Additionally, the possibility of combination with other medications (including antihypertensive and antiarrhythmics) is taken into account.
The initial dose is usually 80 mg, then it is increased (with a minimum interval of 3 days or according to the results of the electrocardiogram) to 160 mg or more. Tablets are taken either 60 minutes before or 2 hours after meals.
Recommended treatment regimen
Sotalex should be taken only with monitoring of the main indicators of the electrocardiogram and renal function.
| Disease | Recommended dosage |
| Initial stages of therapy | Daily dose – 80 mg, taken once or divided into 2 doses with an interval of 12 hours (40 mg each) |
| Prevention of supraventricular arrhythmias | 160 mg twice daily |
| Prevention of arrhythmias during surgery | 120 mg 2 times a day |
| Life-threatening refractory ventricular arrhythmias | 480 mg to 640 mg daily (if potential benefit outweighs perceived risk of adverse effects) |
| Other pathologies | From 160 mg to 320 mg (in 2 doses) |
Possible adverse reactions
Doctors warn about the likelihood of the following side effects:
- bradycardia;
- dyspnea;
- chest pain;
- fatigue;
- drowsiness;
- general weakness;
- headache;
- dry cornea (especially when wearing contact lenses);
- hearing disorders;
- nausea, vomiting, digestive disorders;
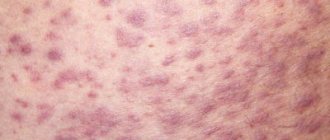
- skin allergic reactions;
- hyperthermia;
- hot flashes and sweating;
- decreased libido, sexual dysfunction;
- depression;
- mood swings.
Muscle weakness, arthro- and myalgia, changes in the lipid profile, and fluctuations in blood sugar levels are also possible.
Possible drug combinations
The combined use of Sotalex is contraindicated with:
- cardiac drugs that prolong the QT interval;
- phenothiazines;
- antidepressants;
- antibiotics intended for intravenous administration;
- β-blockers;
- MAO inhibitors.
Prescribe with caution with:
- calcium channel blockers;
- diuretics, laxatives and other medications that increase the excretion of potassium from the body;
- cardiac glycosides;
- drugs for anesthesia.
When used, individual selection of the dose of hypoglycemic drugs is required. Non-steroidal anti-inflammatory drugs weaken the effect of Sotalex.
Alcohol compatibility
Consumption of alcoholic beverages can cause unpredictable complications.
Special instructions and precautions
Data from clinical studies have demonstrated no risk to humans in terms of genotoxicity and carcinogenicity. However, the drug has pronounced teratogenic properties, slowing down fetal development and causing irreversible neurological disorders associated with the density of β-adrenergic receptors in the central nervous system.
While taking Sotalex, constant monitoring of kidney function is necessary. When the clearance decreases to 30 - 10 ml/min. the daily dose of the medication is reduced by 50%. When this indicator drops to 10 ml/min. - by 75%. If reducing the amount of the drug does not lead to an improvement in kidney condition, treatment is discontinued.
Liver dysfunction does not require medication dosage adjustment.
In some patients, due to prolongation of the QT interval, ventricular tachycardia may develop. The risk of such a complication increases with water-salt imbalance, decompensated heart failure, or a sharp increase in Sotalex dosage.
Dose adjustment may be required for severe diarrhea accompanied by loss of potassium and magnesium. Prescribing the drug to patients after acute myocardial infarction is possible after a thorough diagnosis and assessment of the patient’s condition.
An increase in the QT interval and the development of bradycardia require close monitoring and, if necessary, discontinuation of the drug. The medication is used with caution during exacerbation of allergic rhinitis. There is evidence that Sotalex may enhance the clinical manifestations of psoriasis.
Patients are additionally warned about a possible decrease in the ability to concentrate.
Overdose
Exceeding the dose can be fatal. The patient requires urgent hospitalization with hemodialysis and maintenance therapy.
Storage conditions
At room temperature.
Best before date
Is 3 years.
Discontinuation of therapy
When abruptly stopping taking drugs from the group of β-blockers, almost all patients experience withdrawal syndrome. It manifests itself in the form of an increase in angina attacks, severe arrhythmia, a heart attack, and exacerbation of coronary insufficiency are not excluded. Therefore, the dosage is reduced gradually over 2 - 3 weeks. In some cases, the use of other medications for symptomatic therapy is required.
Sotalex
Monitoring of patients receiving treatment with the drug should include monitoring heart rate and blood pressure (at the beginning of treatment - daily, then once every 3-4 months), ECG, blood glucose concentration in patients with diabetes mellitus (once every 4-5 months ). In elderly patients, it is recommended to monitor renal function (once every 4-5 months). Due to the presence of class III antiarrhythmic properties in the drug, the possibility of prolongation of the QT interval should be monitored and, if necessary, individual dosage selection should be carried out.
For stable angina pectoris, exercise tolerance, the number of angina attacks per day, and the number of nitroglycerin tablets to relieve an angina attack are assessed. The dose of the drug is adequate if the heart rate at rest decreases to 55-60/min, and during exercise - no more than 110/min. It is recommended to use the paired stress test method.
The patient should be taught how to calculate heart rate and instructed about the need for medical consultation if the heart rate is less than 50/min.
Beta blockers are less effective in smokers.
Patients using contact lenses should take into account that during treatment the production of tear fluid may decrease.
Patients with pheochromocytoma are prescribed only after taking an alpha-blocker.
IV administration is possible in the presence of life-threatening tachyarrhythmic heart rhythm disturbances.
IV injections should be carried out slowly under constant monitoring of ECG parameters, respiratory function and blood pressure. If there is a pronounced decrease in blood pressure or a decrease in heart rate, the daily dose should be reduced. Patients with renal failure require dosage adjustment.
In case of thyrotoxicosis, the use of the drug can mask certain clinical signs of thyrotoxicosis (for example, tachycardia). Abrupt withdrawal in patients with thyrotoxicosis is contraindicated because it can increase symptoms.
When prescribing beta-blockers to patients receiving hypoglycemic drugs, caution should be exercised, since hypoglycemia may develop during long breaks in food intake. Moreover, its symptoms such as tachycardia or tremor will be masked due to the action of the drug. Patients should be instructed that the main symptom of hypoglycemia during treatment with beta-blockers is increased sweating.
When taking clonidine concomitantly, it can be discontinued only a few days after Sotalex is discontinued.
It is possible that the severity of the hypersensitivity reaction may increase and the absence of effect from usual doses of epinephrine against the background of a burdened allergic history.
A few days before general anesthesia with chloroform or ether, you must stop taking the drug. If the patient took the drug before surgery, he should select a drug for general anesthesia with minimal negative inotropic effect.
Reciprocal activation of the n.vagus can be eliminated by intravenous administration of atropine (1-2 mg).
Drugs that reduce catecholamine reserves (for example, reserpine) can enhance the effect of beta-blockers, so patients taking such combinations of drugs should be under constant medical supervision to detect arterial hypotension or bradycardia.
Use with caution in combination with psychoactive drugs, such as MAO inhibitors, when taking them on a course for more than 2 weeks.
If elderly patients develop increasing bradycardia (less than 50/min), arterial hypotension (systolic blood pressure below 100 mm Hg), AV block, bronchospasm, ventricular arrhythmias, severe liver and kidney dysfunction, it is necessary to reduce the dose or stop treatment . It is recommended to discontinue therapy if depression caused by taking beta-blockers develops.
Treatment should not be abruptly interrupted due to the risk of developing severe arrhythmias and myocardial infarction. Cancellation is carried out gradually, reducing the dose over 2 weeks or more (reduce the dose by 25% in 3-4 days).
Use during pregnancy and lactation is possible if the benefit to the mother outweighs the risk of side effects in the fetus and child. The drug should be discontinued 48-72 hours before the expected due date.
Avoid ethanol intake during treatment with the drug.
Catecholamines, normetanephrine and vanillylmandelic acid should be discontinued before blood and urine tests; antinuclear antibody titers.
During treatment with the drug, care must be taken when driving vehicles and engaging in other potentially hazardous activities that require increased concentration and speed of psychomotor reactions (individual selection of doses is required for persons whose profession requires these qualities).
Analogs
- Sotahexal (Germany);
- Sotalol (Russia);
- Sotalol Canon (Russia).
In some European countries, Sotalex is sold under the trade name Sotacor (the same manufacturer - Bristol-Myers Squibb).
What is better Sotalex or Sotahexal?
At first glance, Sotagexal is a complete analogue of Sotalex and is also produced in Germany. But, according to experts, the original product from Bristol-Myers Squibb is significantly superior in quality to the generic from Hexal. Patients adapt to treatment more easily and tolerate long-term therapy.
Price and where to buy
The drug is not available in Russia. Pharmacies offer only generics and domestically produced analogues. But there are intermediary companies that organize the purchase and delivery of medicines from Europe. After placing an order by phone or on the website of the online pharmacy, Sotalex will be quickly delivered from Germany to Moscow or St. Petersburg, and from there throughout Russia.
The cost of a package of 100 tablets (regardless of the dosage) is approximately 75 - 80 euros. If you order several pieces at once, the price will be discounted. The manager will tell you exactly how much Sotalex costs including delivery.
Pharmacies in Moscow where you can buy Sotalex (Sotalol), compare prices and make a pre-order
Composition and release form
Tablets 1 tablet. sotalol hydrochloride 160 mg excipients: lactose monohydrate; stearic acid; magnesium stearate; corn starch; anhydrous colloidal silicon dioxide; MCC
10 pcs in blister pack; There are 3 blisters in a box. pharmachologic effect
Antiarrhythmic.
Non-selective beta-blocker, acts on β1- and β2-adrenergic receptors, belongs to class III antiarrhythmic drugs. It has a pronounced antiarrhythmic effect, the mechanism of which is to increase the duration of the action potential and lengthen the absolute refractory period in all parts of the conduction system of the heart. Reduces heart rate and myocardial contractility, slows down AV conduction. Increases the tone of bronchial smooth muscles.
Indications
Supraventricular tachycardia (including with WPW syndrome), paroxysmal form of atrial fibrillation, ventricular tachycardia.
Contraindications
Acute heart failure or decompensated chronic heart failure, cardiogenic shock, AV block II or III degree, sinoatrial block, SSS, sinus bradycardia (heart rate less than 55 beats/min), Prinzmetal's angina, cardiomegaly (without signs of heart failure), arterial hypotension (systolic blood pressure less than 90 mm Hg, especially with myocardial infarction); COPD, bronchial asthma (severe); occlusive diseases of peripheral vessels (complicated by gangrene, intermittent claudication or pain at rest), diabetes mellitus with ketoacidosis, metabolic acidosis, concomitant use of MAO inhibitors, lactation, hypersensitivity to sotalol and sulfonamides.
Directions for use and doses
Inside, 1–2 hours before meals.
At the beginning of treatment, it is recommended to take 160 mg per day in 2 divided doses (about 12 hours apart). This dose can be increased if necessary after appropriate clinical assessment of the patient's condition to 240 or 320 mg per day. In most patients, a therapeutic response is achieved at a total daily dose of 160–320 mg, divided into 2 doses. Some patients with life-threatening refractory ventricular arrhythmia may require up to 480–640 mg of the drug per day; however, such doses can be prescribed only in cases where the potential benefit outweighs the increased risk of side effects, especially proarrhythmogenic effects. In patients with cardiomyopathy or chronic heart failure with angina pectoris, arterial hypertension, and conditions after myocardial infarction, it is recommended to begin treatment in a hospital setting. The initial dose is 160 mg per day in 1 or 2 divided doses. After a week, the dose can be increased, if necessary, by 80 mg/day (1/2 tablet of 160 mg each) at weekly intervals. The rate of dose increase depends on the patient’s tolerability of the drug, which, in particular, is assessed by the degree of induced bradycardia and clinical response. Due to the relatively long T1/2 in most patients, Sotalex® is effective when taken once a day. The dose interval is 160–320 mg/day.
Use in patients with impaired renal function. Since sotalol is excreted from the body mainly in the urine, and its T1/2 increases in the presence of renal failure, the dosage of the drug should be reduced when the serum creatinine level is more than 120 μmol/L in accordance with the following recommendations: Serum creatinine Recommended dose μmol/L mg /dl less than 120 less than 1.2 usual dose 120–200 1.20 — less than 2.3 3/4 the usual dose 200–300 2.3 — less than 3.4 1/2 the usual dose 300–500 3.4 — less 5.7 1/4 usual dose Storage conditions
At a temperature of 15–25 °C.
Keep out of the reach of children. Best before date
3 years.