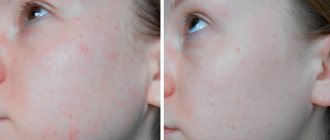
Medicines from this manufacturer are suitable for the treatment of 1st and 2nd degree burns and any other non-infected skin lesions (including the treatment of sunburn).
Biafine is produced in three forms - cream, ointment and emulsion. They work on the skin by providing intense hydration to optimize healing processes and protect wounds from contamination.
These medications are used for a variety of ulcerative lesions of the skin surface, for inflammatory phenomena provoked by radiation therapy, for various wounds and cuts as soft healing preparations.
Biafine cream and ointment have a more concentrated composition compared to the emulsion. They are recommended to be applied to wounds and various areas of ulcerative skin lesions. The emulsion is used when treating large areas of the body, for example, for sunburn.
pharmachologic effect
Thanks to the main components specified in the instructions for use of this drug, the medication has a regenerative effect on the skin. It heals wounds, promotes nutrition and hydration of cellular structures after various burns and other skin damage. This pharmacological agent is not used for infected and purulent wounds, since the drug does not have antiseptic properties and does not have a bactericidal effect.
Interaction with other medications
The active substance of the drug is processed in the liver. The dosage should be calculated correctly when prescribing Binafin simultaneously with other drugs that have the same method of elimination. The tablet form of terbinafine affects the metabolism of a number of antidepressants and antihypertensive drugs, accelerating their elimination.
The patient must inform the doctor if he is taking:
- Amitriptyline;
- Ludiomil;
- Pyrazidol;
- Paxil;
- Fluoxetine;
- cardioselective adrenergic blockers.
This is important if drugs for long-term treatment are recommended in low dosages. The patient may notice a decrease in the effectiveness of antihypertensive and antidepressant therapy.
Cimetidine slows down and rifampicin accelerates the excretion of Binafin, which leads to the need to adjust the dose of the antifungal agent. When prescribed with birth control pills, in rare cases, fluctuations in the duration of the menstrual cycle occur. The interaction of the cream with other products has not been registered.
Contraindications
The use of Biafine emulsion and cream is not recommended in the following cases:
- in case of allergic reactions to any ingredient in the composition;
- bleeding wounds;
- the occurrence of a skin rash of an allergic nature.
The drug is used with caution in the following situations:
- pregnancy, lactation;
- allergy;
- wounds or other skin lesions of an infectious nature;
- period of radiation therapy.
Application and dosage
Before using Biafine ointment, emulsion, cream, you must wash your hands thoroughly. If superficial burns occur, as well as minor sunburn, apply the product in a thick layer to the treated area 2 to 4 times a day. When treating wounds and abrasions, it is recommended to pre-clean with soap and water, thoroughly rinse the surface and apply the product in a thick layer, including outside the lesions.
If this product is used for therapeutic measures to treat the skin after radiation therapy, it must be applied with light massaging movements until completely absorbed. This is confirmed by the instructions for the Biafin emulsion.
The duration of treatment usually depends on the degree and severity of the pathological condition, but in most cases it takes approximately 1 month.
Overdose
Several cases of overdose with Binafin tablets have been described. Affected patients used up to 3 g per day (from the recommended 0.25 g). An overdose is indicated by the appearance of symptoms such as stomach pain, attacks of nausea, and a sharp headache accompanied by dizziness.
Binafin for nail fungus in overdose can cause severe headache and dizziness.
To alleviate the condition, you need to take activated charcoal to absorb the drug, rinse your stomach, and visit a dermatologist to monitor the condition and decide on further therapy.
Cases of overdose of the cream when used correctly have not been described.
Side effects
The occurrence of adverse reactions as a result of the use of this medicine may be due to the properties of the medicinal compounds present in its composition. Among them, the following pathological phenomena are most often observed:
- itching, tingling after application;
- symptoms of skin irritation (due to the presence of propylene glycol);
- allergic phenomena, contact dermatitis.
Since the components of the cream and emulsion do not have the ability to penetrate the bloodstream, there is no interaction of the product with other medications.
If a beneficial effect of treatment is not observed within two weeks, it is recommended to consult a specialist.
For liver dysfunction
Due to the lack of clinical studies of the drug in patients with liver diseases, the administration of the tablet form is contraindicated. There is information about the occurrence of liver failure with a severe outcome while taking Binafin, but a cause-and-effect relationship with taking the drug has not been proven.
If liver pathology is suspected, the patient should closely monitor his well-being during the course of treatment, and at least once every 4 weeks, evaluate biochemical blood parameters under the supervision of a dermatologist.
Reviews of Biafine cream, ointment, emulsion
The drug is not very popular on the domestic pharmacological market, and this is not surprising, since it is extremely difficult to obtain the necessary information about this drug on the Internet - there is very little of it. From the few reviews it is known that patients who used this medication did not receive proper treatment for skin pathologies and, in their opinion, this is an absolutely useless drug.
In most cases, it did not cause side effects, but the therapeutic effect from its use was minimal. Many of the patients indicated that they would not recommend this drug to their relatives and friends, but they themselves decided to use other, more effective medications.
There are virtually no positive reviews of Biafine cream, except for those cases where people have used it as a treatment for skin redness after prolonged exposure to the sun.
Binafin cream 1% 10g No. 1 28450
Description
Binafin Shreya Life Sciences (India) cream for external use 1%; tube 10 g, cardboard pack 1; EAN code: 8906000574058; No. P N014467/02-2002, 2008-11-17 from Shreya Life Sciences (India) Latin name Binafin Active ingredient Terbinafine * (Terbinafinum) ATX: D01AE15 Terbinafine Pharmacological group Antifungal agent [Antifungal agents] Nosological classification (ICD-10) B35 .4 Mycosis of the trunk B36.0 Lichen versicolor B36.9 Superficial mycosis, unspecified B37.2 Candidiasis of the skin and nails Description of the dosage form Tablets, 125 mg. White to white with a yellowish tint, round, biconvex. Tablets, 250 mg. White to white with a yellowish tint, round, flat, with cut edges. Cream. White homogeneous cream of soft consistency. Pharmacological action Pharmacological action - broad spectrum antifungal. Pharmacodynamics Terbinafine is an allylamine that has a broad spectrum of action against fungi that cause diseases of the skin, hair and nails, incl. dermatophytes such as Trichophyton (e.g. T.rubrum, T.mentagrophytes, T.tonsurans, T.verrucosum, T.violaceum), Microsporum (e.g. M. canis), Epidermophyton floccosum, as well as yeast-like fungi of the genus Candida (e.g. Candida albicans) and Pityrosporum. At low concentrations, terbinafine has a fungicidal effect against dermatophytes, molds and some dimorphic fungi. Activity against yeast-like fungi, depending on their type, can be fungicidal or fungistatic. Terbinafine specifically inhibits the early stage of sterol biosynthesis in the fungal cell. This leads to ergosterol deficiency and intracellular accumulation of squalene, which causes the death of the fungal cell. Terbinafine works by inhibiting the enzyme squalene epoxidase in the cell membrane of the fungus. This enzyme does not belong to the cytochrome P450 system. Terbinafine does not affect the metabolism of hormones or other drugs. When Binafin is administered orally, concentrations of the drug are created in the skin, hair and nails, providing a fungicidal effect. Pharmacokinetics After a single oral dose of terbinafine at a dose of 250 mg, Cmax of the drug in blood plasma is reached after 2 hours and is 0.97 mcg/ml. The half-life of absorption is 0.8 hours; and the half-life of distribution is 4.6 hours. No dose adjustment is required when taken simultaneously with food. Terbinafine is highly bound to plasma proteins (99%), quickly penetrates the dermal layer of the skin and is concentrated in the lipophilic stratum corneum. Terbinafine also penetrates the secretions of the sebaceous glands, which leads to the creation of high concentrations in the hair follicles, hair and skin rich in sebaceous glands. Terbinafine has also been shown to penetrate the nail plates in the first few weeks after initiation of therapy. Terbinafine is metabolized quickly and substantially with the participation of at least seven isoenzymes of cytochrome P450, with the main role being played by the isoenzymes CYP2C9, CYP1A2, CYP3A4, CYP2C8 and CYP2C19. As a result of the biotransformation of terbinafine, metabolites are formed that do not have antifungal activity and are excreted primarily in the urine. The final T1/2 of the drug is 17 hours. There is no evidence of cumulation of the drug in the body. There were no changes in Binafine plasma Css depending on age, but in patients with impaired renal or hepatic function, the rate of elimination of the drug may be slowed, resulting in higher plasma concentrations of terbinafine. In pharmacokinetic studies of a single dose of Binafin in patients with concomitant liver diseases, the possibility of reducing drug clearance by 50% was shown. Indications for the drug Binafin Tablets: onychomycosis caused by dermatophyte fungi; mycoses of the scalp; fungal infections of the skin - treatment of dermatomycosis of the trunk, legs, feet, as well as yeast infections of the skin caused by fungi of the genus Candida (for example, Candida albicans) - in cases where the localization, severity or prevalence of the infection determine the advisability of oral therapy. Cream fungal skin infections caused by dermatophytes such as Trichophyton (eg T.rubrum, T.mentagrophytes, T.verrucosum, T.violaceum, T.interdigitale, T. tonsurans, T. gipseum), Microsporum canis and Epidermophyton floccosum; yeast infections of the skin, mainly those caused by the genus Candida (eg Candida albicans); Versicolor versicolor (Pityriasis versicolor), caused by Pityrosporum orbiculare (also known as Malassezia furfur). Contraindications Hypersensitivity to terbinafine or any other component included in the tablets or cream. Use during pregnancy and breastfeeding Tablets. Since clinical experience with the use of Binafin in pregnant women is very limited, the drug should not be used during pregnancy unless the potential therapeutic effect outweighs the possible risk of therapy. Terbinafine is excreted in breast milk, so women receiving Binafin by mouth should not breastfeed. Cream. The drug should be used during pregnancy only for very strict indications and if the expected benefit to the mother outweighs the potential risk to the fetus and child. If it is necessary to prescribe the drug during lactation, the issue of stopping breastfeeding should be decided. Side effects Tablets. Binafin is generally well tolerated. Side effects are usually mild or moderate and are transient. Most often (with a frequency of 1 to 10%), symptoms from the gastrointestinal tract (feeling of fullness in the stomach, loss of appetite, dyspepsia, nausea, mild abdominal pain, diarrhea), mild skin reactions (rash, urticaria), musculoskeletal reactions are observed. (arthralgia, myalgia). With a frequency of 0.1 to 1%, taste disturbances occur, including their loss (recovery occurs within a few weeks after cessation of treatment); rarely (with a frequency of 0.01 to 0.1%) hepatobiliary disorders (primarily associated with cholestasis, including cases of liver failure) have been reported in connection with treatment with Binafin. There have been reports of cases of liver failure, some of which resulted in death or liver transplantation, but in most cases the patients had serious concomitant diseases, and the relationship of cases of liver failure with the use of Binafin was regarded as doubtful. There are reports of very rare (with a frequency of less than 0.01%) serious skin reactions - Stevens-Johnson syndrome, toxic epidermal necrolysis, anaphylactoid reactions. If a progressive skin rash develops, treatment with Binafin should be discontinued. There are also reports of very rare hematological disorders, such as neutropenia, agranulocytosis, thrombocytopenia. There are reports of very rare cases of hair loss, although the causal relationship of this phenomenon with taking the drug has not been established. Cream. In rare cases, redness, itching or burning sensation was observed at the site of application of the drug, but these phenomena rarely led to the need to stop treatment. The above symptoms should be distinguished from allergic reactions (for example, urticaria), which occur rarely, but if they occur, treatment should be stopped. Interaction Tablets. Results from in vitro studies in healthy volunteers indicate that terbinafine has little potential to inhibit or enhance the clearance of most drugs that are metabolized by the cytochrome P450 system (e.g. cyclosporine, terfenadine, tolbutamide, triazolam or oral contraceptives). In vitro studies, however, showed that terbinafine inhibits CYP2D6-mediated metabolism. These data may be clinically significant for those drugs that are predominantly metabolized by this enzyme, such as tricyclic antidepressants, beta-blockers, SSRIs and MAO B inhibitors (if the concomitant drug has a small therapeutic concentration range). In patients who simultaneously took Binafin and oral contraceptives, in some cases, irregular menstrual cycles were noted, although the frequency of these irregularities remained within the range observed in patients using only oral contraceptives. On the other hand, the overall clearance of terbinafine may be accelerated by drugs that accelerate metabolism (such as rafampicin) and may be slowed by drugs that inhibit cytochrome P450 (such as cimetidine). In cases where simultaneous use of these drugs is necessary, adequate adjustment of the dose of Binafin may be required. Cream. Currently, drug interactions are unknown. Overdose Symptoms: headache, dizziness, nausea, pain in the epigastric region. Treatment: gastric lavage, administration of activated carbon, symptomatic therapy. Special instructions Tablets. If during treatment with Binafin the patient experiences phenomena that suggest liver dysfunction, such as unexplained persistent nausea, vomiting, lack of appetite, fatigue, jaundice, pain in the right hypochondrium, dark urine or discolored feces, in this case the hepatic origin of these symptoms should be confirmed (determining serum concentrations of ALT, AST) and discontinue treatment with Binafin. The patient should be warned about the need to consult a doctor if such symptoms occur. Since prospective clinical studies have not been conducted to study the course use of Binafin in patients with concomitant chronic or active liver diseases, its use in this group of patients is not recommended. Patients with impaired renal function (Cl creatinine <50 ml/min or serum creatinine level >300 µmol/l) should receive half the usual dose of the drug. In vitro studies have shown that terbinafine inhibits metabolism mediated by the cytochrome 2D6 enzyme (CYP2D6). Therefore, it is necessary to constantly monitor patients receiving concomitant treatment with Binafin with drugs that are predominantly metabolized with the participation of this enzyme, such as tricyclic antidepressants, beta-blockers, SSRIs and MAO type B inhibitors, if the drug used simultaneously has a small therapeutic range concentration. Impact on the ability to drive a car and operate machinery. There is no data on the effect of Binafin on the ability to drive a car and operate machinery. Cream. Intended for outdoor use only. Avoid contact of the drug with the eyes. Release form Tablets, 125, 250 mg. For 10, 14, 20 tables. in a blister made of PVC-aluminum foil. 1 or 2 blisters are placed in a cardboard box. Cream, 1%. In tubes of 10, 15, 30 g. Manufacturer: Shreya Life Sciences Pvt. Ltd., India. Shreya House, 301/A, Pereira Hill Road, Andheri (East), Mumbai - 400 099, India. Send consumer claims to the representative office address 111033, Moscow, st. Zolotorozhsky Val, 11, building 21. Tel.. Conditions for dispensing from pharmacies Tablets. On prescription. Cream. Over the counter. Storage conditions for the drug Binafin: In a dry place, at a temperature of 2–30 °C. Keep out of the reach of children. The shelf life of the drug Binafin is 3 years. 2000-2014. Register of Medicines of Russia The database is intended for healthcare professionals. Commercial use of materials is not permitted.