Write a review
Reviews: 0
Manufacturers: Schering Wien Ges.mbH
Active ingredients
- Estradiol
Disease class
- Ovarian dysfunction
- Primary ovarian failure
- Postmenopausal osteoporosis
- Absence of menstruation, scanty and infrequent menstruation
- Dysmenorrhea, unspecified
- Menopause and menopause in women
Clinical and pharmacological group
- Not indicated. See instructions
Pharmacological action
- Antimenopausal
Pharmacological group
- Estrogens, gestagens; their homologs and antagonists in combinations
Composition of the drug and release form
The drug Cyclo-Proginova is presented as a set of two types of dragees with different compositions of components.
One white dragee contains:
- Active ingredients: 2 mg estradiol (as valerate)
- Auxiliary ingredients included in the core: 26.2 mg corn starch, 46.25 mg lactose monohydrate, 0.15 mg E572, 3 mg povidone, 2.4 mg talc
- Dragee coating ingredients: 75 mcg wax, 14.71 mg calcium carbonate, 3.77 mg macrogol-6000, 296 mcg povidone, 33.54 mg sucrose.
One pale brown dragee contains:
- Active ingredients: 2 mg estradiol (as valerate), 0.5 mg norgestrel
- Auxiliary ingredients that make up the dragee core: 46.75 mg lactose (as monohydrate), 26.2 mg corn starch, 3 mg povidone, 2.4 mg talc, 150 mcg E572
- Dragee coating ingredients: 33.43 mg sucrose (as monohydrate), 204 mcg glycerol, 14.54 mg calcium carbonate, 102 mcg E172 yellow dye, 123 mcg E172 red dye, 3.71 mg macrogol-6000, 0.323 mg povidone, 7.09 mg talc, 408 mcg E171, 75 mcg wax.
The Cyclo Proginova set contains 11 white and 10 pale brown dragees. Both types of pills are placed in one blister with a calendar index. The cardboard package contains one tablet of dragee and an accompanying instruction manual.
Medicinal properties
The therapeutic effect of Cyclo-Proginov is achieved due to the active compound present.
Estradiol valerate replaces missing endogenous estrogens. The substance also takes part in many important processes: carbohydrate, protein and fat metabolism, normalizes cholesterol levels (reducing the level of “bad” and increasing the content of good). The substance is necessary for the formation of sexual characteristics when they are insufficiently developed.
The use of the substance for the treatment of women during menopause is due to the ability of the synthetic hormone to improve the condition of bone tissue and prevent the development of osteoporosis. In addition, estradiol has a positive effect on the nervous system, relieving disorders and symptoms of natural or medical menopause.
After penetration into the body, the substance is absorbed into the gastrointestinal tract at high speed and then converted into metabolites. Peak concentrations occur within 3-6 hours. The substance leaves the body along with urine.
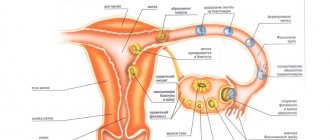
For women of childbearing age, Cyclo-Proginova helps normalize MC by adjusting its duration and intensity.
The second substance included in the pale brown pills is norgestrel, an artificially synthesized gestagen. The substance is similar in properties to pregesterone, the endogenous hormone of the corpus luteum, but exceeds it in potency. Stimulates the transition of the mucous membranes of the uterus to the stage of secretion. Suppresses the formation of gonadotropes, blocks ovulation.
Mode of application
The average price of a pack (21 tablets) is 817 rubles.
The instructions for use recommend that you start taking Cyclo-Proginov tablets on the 5th day of the MC. Course – until the 25th day. Women with infrequent periods or amenorrhea, as well as postmenopausal patients, are allowed to choose any day of their appointment. The main condition for starting the course is a confirmed absence of pregnancy.
The patient can choose her own appointment time, but after that she should stick to the same appointment hours. The manufacturer's instructions recommend taking the tablets in the morning or evening according to the schedule indicated on the calendar scale of the blister. It is not recommended to chew or bite the pills - only swallow them whole with a small amount of water. First, white pills are taken, then pale brown ones.
After finishing one tablet of pills, you need to take a week break. During this period, bleeding similar to menstruation should occur - usually this happens from the 2nd to the 4th day of the interval.
If the doctor has not made a different prescription, then, in accordance with the instructions, after the end of the week's respite, they begin to take the pills from the new blister.
The appointment will need to be postponed if bleeding has not occurred within a week. In this case, you should make sure that there is no or no pregnancy. A new course can be started only after its exclusion.
During pregnancy and pregnancy
The drug is prohibited for use during pregnancy. If pregnancy occurs during a course of pills, then the drug must be discontinued immediately.
Lactating women should also not use Cyclo-Proginova, since the hormones it contains can pass into the milk.
Similar drugs:
- Abufene Oral tablets
- Angeliq Tablets
- Klimonorm Dragee
- Klimadynon Drops for oral administration
- Klimadynon Uno Oral tablets
- Climodien Oral tablets
- Klimadynon Oral tablets
- Climen Dragee
** The Drug Directory is intended for informational purposes only. For more complete information, please refer to the manufacturer's instructions. Do not self-medicate; Before starting to use the drug Cyclo-Proginova, you should consult a doctor. EUROLAB is not responsible for the consequences caused by the use of information posted on the portal. Any information on the site does not replace medical advice and cannot serve as a guarantee of the positive effect of the drug.
Are you interested in the drug Cyclo-Proginova? Do you want to know more detailed information or do you need a doctor's examination? Or do you need an inspection? You can make an appointment with a doctor - the Euro lab is always at your service! The best doctors will examine you, advise you, provide the necessary assistance and make a diagnosis. You can also call a doctor at home . Euro lab clinic is open for you around the clock.
** Attention! The information presented in this medication guide is intended for medical professionals and should not be used as a basis for self-medication. The description of the drug Cyclo-Proginova is provided for informational purposes and is not intended for prescribing treatment without the participation of a doctor. Patients need to consult a specialist!
If you are interested in any other drugs and medications, their descriptions and instructions for use, information about the composition and form of release, indications for use and side effects, methods of use, prices and reviews of drugs, or you have any other questions and suggestions - write to us, we will definitely try to help you.
Contraindications
The hormonal drug Cyclo-Proginova is prohibited from use if the patient has one or more of the following factors. If during the course the condition worsens or an illness occurs, the medicine should be discontinued immediately. The grounds for prohibiting therapy with the participation of Cyclo-Proginov are:
- Pregnancy and breastfeeding
- Undiagnosed vaginal bleeding
- Diagnosed or suspected breast cancer
- Identified or suspected hormone-dependent cancer or malignant neoplasms activated under the influence of hormones
- Liver neoplasms at the time of prescription of Cyclo-Proginov or those present in the medical history
- Complex liver pathologies
- Acute pulmonary embolism (such as stroke or MI)
- Hereditary or acquired predisposition to the development of thrombosis of veins and arteries
- Presence of risk factors for thrombosis
- Exacerbation of DVT
- Current or history of pulmonary embolism
- Hypertriglyceridemia
- Individual hypersensitivity to drug ingredients
- Congenital deficiency of lactase in the body, deficiency of sucrase and/or isomaltose, lactose intolerance, GG malabsorption syndrome
- Children and adolescence (up to 18 years).
Prescribing Cyclo-Proginov is possible, but only after a thorough examination and study of the benefit/harm ratio for:
- Hypertension
- Hereditary hyperbilirubinemia
- Intrahepatic cholestasis
- Presence of cholestatic itching during previous pregnancy
- Endometriosis
- Fibromyoma
- Diabetes mellitus.
special instructions
Cycloproginova is not used as a contraceptive against unwanted pregnancy and for this you need to use other non-hormonal methods. In addition, if pregnancy is suspected, taking the pills should be stopped until this fact is clarified in order to avoid complications and risks to the fetus in the future. Cycloprogynova requires careful use when:
- Thrombotic disorders;
- Suspicion of stroke, development of venous or arterial thromboembolism;
- Endometrial cancer, breast cancer;
- Development of a tumor in the liver;
- Gallstone disease;
- The appearance of severe headaches;
- Arterial hypertension;
- Damage to liver functions;
- Development of hyperbilirubinemia;
- Increase in size of the uterus.
In addition, treatment should be stopped; endometriosis began to recur while taking HRT, and general health worsened while taking the pills. A complete examination of the mammary glands, cytology of cervical mucus to rule out pregnancy and possible bleeding disorders, and suspected thromboembolic disorders are required.
Precautionary measures
If the patient has a predisposition to thrombosis or had previous conditions that increase the threat of its development, then it is not advisable to use Cyclo-Proginova. If you prescribe hormonal drugs, you must adhere to the following recommendations:
- Medical examinations
Before starting the Cyclo-Proginova course, the patient should be examined by a doctor, and then during treatment regularly check her condition (once every six months, including a visit to the gynecologist and a mammographic examination). This is especially important for women with pituitary adenoma.
- Risk factors for HRT
Considering that various complications may arise during replacement therapy, a woman should inform doctors about the presence of the following diseases or conditions:
- Fibroids
- Presence of endometriosis at the time of prescription of Cyclo-Proginov or in the medical history
- Pathologies of the liver and gallbladder (if a woman had hepatitis, then you can start HRT six months after treatment)
- Jaundice during pregnancy or after hormone therapy
- Diabetes
- Hypertension
- Hyperpigmentation (including in the past)
- Mastopathy
- BA
- Migraine
- Porphyrin disease
- Otospongiosis
- KS lupus
- Risk of blood clots due to age, surgery, obesity, varicose veins, prolonged immobility.
Oncology
When treating Cyclo-Proginov, as with any HRT drug, possible adverse events should be taken into account:
During hormone therapy, the risk of endometrial cancer increases, so additional use of progestin drugs may be required. If the circulatory system is disrupted or breakthrough bleeding develops, it is necessary to check with a gynecologist.
According to medical research, using HRT for several years increases the risk of breast cancer.
The formation of liver tumors (benign and malignant) is possible, which can provoke internal bleeding and fatal consequences. Therefore, if unusual sensations appear, you should immediately consult a doctor.
- Cholelithiasis
There is evidence that estrogens can negatively affect the state of bile. Therefore, in some patients, the use of Cyclo-Proginov may contribute to the development of cholelithiasis.
Factors for emergency withdrawal of Cyclo-Proginov:
- Migraine: if it occurs for the first time, in the case of habitual migraines – an unusually severe manifestation
- Unexplained hearing and/or vision loss
- Venous inflammation
- If signs of thrombosis appear: sudden cough (may be with blood), swelling of the legs, pain in the limbs, unexplained deterioration in breathing, fainting
- Pregnancy.
Dragee Cyclo-Proginova
Instructions for medical use of the drug
Description of pharmacological action
Replenishes insufficient production of endogenous estrogens, reduces LDL cholesterol levels in the blood. Relieves somatic, mental and other menopausal symptoms during pre- and postmenopausal periods; prevents loss of bone mass and osteoporosis. Hormone replacement therapy reduces the risk of developing cardiovascular diseases; in combination with gestagen - prevents the development of proliferative processes in the endometrium.
Indications for use
Estrogen deficiency (pre- and postmenopausal period, oophorectomy for non-malignant tumors, radiation castration); prevention of osteoporosis in postmenopause, amenorrhea (primary and secondary), ovarian dysfunction.
Release form
dragee; blister 21 packs cardboard 1; Composition of Dragee 1 set White dragee 1 dragee estradiol valerate 2 mg Light brown dragee 1 dragee estradiol valerate 2 mg norgestrel 0.15 mg excipients: lactose monohydrate; corn starch; povidone 25000; talc; magnesium stearate; crystalline sucrose; povidone 700000, macrogol 6000; calcium carbonate; wax montanglycol Additionally for light brown dragees - glycerol 85%; calcium carbonate precipitated; titanium dioxide; iron oxide yellow pigment; red iron oxide in a blister with a calendar scale of 21 tablets (11 white and 10 light brown); 1 blister in a box.
Pharmacodynamics
Cyclo-Proginova contains estrogen - estradiol valerate, which in the human body is converted into natural 17β-estradiol. Cyclo-Proginova also contains a progesterone derivative, norgestrel. Adding norgestrel for 10 days of each cycle prevents the development of endometrial hyperplasia and cancer. Thanks to the composition and cyclic regimen of Cyclo-Proginov (taking only estrogen for 11 days, then a combination of estrogen and gestagen for 10 days, and finally a 7-day break), a menstrual cycle is established in women with an unremoved uterus when taking the drug regularly . While taking Cyclo-Proginov, ovulation is not suppressed, and the production of hormones in the body itself remains virtually unchanged. Cyclo-Proginova can be used by women of reproductive age to regulate the menstrual cycle, as well as by perimenopausal women to treat irregular uterine bleeding. Estradiol replenishes the deficiency of estrogen in the female body after menopause and provides effective treatment of psycho-emotional and autonomic menopausal symptoms (such as hot flashes, increased sweating, sleep disturbance, increased nervous excitability, irritability, palpitations, cardialgia, dizziness, headache, decreased libido, muscle and joint pain); involution of the skin and mucous membranes, especially the mucous membranes of the genitourinary system (urinary incontinence, dryness and irritation of the vaginal mucosa, pain during sexual intercourse). Estradiol prevents bone loss caused by estrogen deficiency. This is mainly due to the suppression of osteoclast function and a shift in the process of bone formation towards bone formation. Long-term use of hormone replacement therapy (HRT) has been shown to reduce the risk of peripheral bone fractures in postmenopausal women. When HRT is discontinued, the rate of bone mass decline is comparable to that characteristic of the period immediately after menopause. It has not been proven that using HRT can restore bone mass to premenopausal levels. HRT also has a beneficial effect on the collagen content of the skin, as well as its density, and may also slow down the formation of wrinkles. HRT leads to a decrease in total cholesterol, low-density lipoprotein (LDL) cholesterol and an increase in high-density lipoprotein (HDL) cholesterol, as well as an increase in triglyceride levels. The gestagen contained in Cyclo-Proginova to a certain extent prevents the effects of estradiol on lipid metabolism. Observational studies suggest that among postmenopausal women, the incidence of colon cancer is reduced when using HRT. The mechanism of action is still unclear.
Pharmacokinetics
After oral administration of estradiol valerate is quickly and completely absorbed from the gastrointestinal tract. After entering the body, it is rapidly metabolized to form 17β-estradiol and estrone, which subsequently undergo standard metabolic transformations. After oral administration, the bioavailability of estradiol is about 3%. Food intake does not affect the bioavailability of estradiol. The maximum serum estradiol concentration of approximately 30 pg/ml is usually achieved 4-9 hours after taking the tablets. Estradiol is excreted in the form of metabolites, mainly in the urine in the form of sulfates and glucuronides. After oral administration, norgestrel is quickly and almost completely absorbed from the gastrointestinal tract. The maximum serum concentration of levonorgestrel, approximately 7-8 pg/ml, is usually achieved 1-1.5 hours after taking the tablets. Levonorgestrel binds to albumin and sex hormone binding globulin (SHBG). About 1-1.5% of the total serum concentration of levonorgestrel is not protein bound. With a half-life of approximately 1 day, norgestrel metabolites are excreted in urine and bile.
Use during pregnancy
HRT is not prescribed during pregnancy or breastfeeding. Large-scale epidemiological studies of steroid hormones used for contraception or HRT have found no increased risk of birth defects in children born to women who took such hormones before pregnancy, or teratogenic effects of the hormones when accidentally taken during early pregnancy. Small amounts of sex hormones can be excreted in breast milk.
Other special occasions at reception
Contraindicated in patients with a current or history of liver tumor (benign or malignant); for severe liver diseases.
Contraindications for use
It is not recommended to start hormone replacement therapy (HRT) if you have any of the following conditions. If any of these conditions occur during HRT, you should immediately stop using the drug. — pregnancy and lactation; - vaginal bleeding of unknown origin; - confirmed or suspected diagnosis of breast cancer; — confirmed or suspected diagnosis of a hormone-dependent precancerous disease or hormone-dependent malignant tumor; - liver tumors currently or in history (benign or malignant); - severe liver diseases; - acute arterial thrombosis or thromboembolism (such as myocardial infarction, stroke); — deep vein thrombosis in the acute stage, thromboembolism currently or in history; - severe hypertriglyceridemia; - hypersensitivity to the components of the drug Cyclo-Proginova. With caution: Cyclo-Proginova should be prescribed with caution for the following diseases: - arterial hypertension; - congenital hyperbilirubinemia (Gilbert, Dubin-Johnson and Rotor syndromes); - cholestatic jaundice or cholestatic itching during pregnancy; - endometriosis; - uterine fibroids; — diabetes mellitus (see “Special Instructions”).
Side effects
From the reproductive system and mammary glands: changes in the frequency and intensity of uterine bleeding, breakthrough bleeding, intermenstrual bleeding (usually weakened during therapy), dysmenorrhea, changes in vaginal discharge. a condition similar to premenstrual syndrome; tenderness, tension and/or enlargement of the mammary glands. From the gastrointestinal tract: dyspepsia, bloating, nausea, vomiting, abdominal pain, relapse of cholestatic jaundice. From the skin and subcutaneous tissue: skin rash, itching, chloasma, erythema nodosum. From the central nervous system: headache, migraine, dizziness, anxiety or depressive symptoms, increased fatigue. Other: rapid heartbeat, edema, increased blood pressure, venous thrombosis and thromboembolism, muscle cramps, changes in body weight, changes in libido, visual disturbances, intolerance to contact lenses, allergic reactions.
Directions for use and doses
If the patient is still menstruating, treatment should begin on day 5 of the menstrual cycle (day 1 of menstrual bleeding corresponds to day 1 of the menstrual cycle). Patients with amenorrhea or very infrequent menstruation, as well as postmenopausal women, can start taking the drug at any time, provided that pregnancy is excluded (see section "Pregnancy and lactation"). Each package is designed for 21 days of use. Take one white pill every day for the first 11 days, and then one light brown pill every day for 10 days. After 21 days of taking the drug, there is a 7-day break in taking the drug, during which menstrual-like bleeding occurs due to drug withdrawal (usually 2-3 days after taking the last pill). After a 7-day break from taking the drug, start a new package of Cyclo-Proginova, taking the first tablet on the same day of the week as the first tablet from the previous package. The dragees are swallowed whole with a small amount of liquid. The time of day when a woman takes the drug does not matter, however, if she started taking the pills at a specific time, she should stick to that time further. If a woman forgot to take the pill, she can take it within the next 12-24 hours. If treatment is interrupted for a longer time, vaginal bleeding may occur.
Overdose
There is no risk of serious side effects when accidentally taking the drug Cyclo-Proginova in an amount many times higher than the daily therapeutic dose. Symptoms that may occur in case of overdose: nausea, vomiting, vaginal bleeding. There is no specific antidote, treatment is symptomatic.
Interactions with other drugs
When starting HRT, you must stop using hormonal contraceptives. If necessary, the patient should be advised to use non-hormonal contraceptives. Long-term treatment with drugs that induce liver enzymes (for example, some anticonvulsants and antimicrobial drugs) may increase the clearance of sex hormones and reduce their clinical effectiveness. A similar property to induce liver enzymes has been found in hydantoins, barbiturates, primidone, carbamazepine and rifampicin, and the presence of this feature is also suggested in oxcarbazepine, topiramate, felbamate and griseofulvin. Maximum enzyme induction is usually observed no earlier than 2-3 weeks, but it may then persist for at least 4 weeks after discontinuation of the drug. In rare cases, during concomitant use of certain antibiotics (for example, penicillin and tetracycline groups), a decrease in estradiol levels was observed. Substances that are highly conjugated (for example, paracetamol) may increase the bioavailability of estradiol due to competitive inhibition of the conjugation system during absorption. Due to the effect of HRT on glucose tolerance, the need for oral antidiabetic agents or insulin may change in some cases. Interaction with alcohol: Excessive alcohol consumption during HRT may increase circulating estradiol levels.
Special instructions for use
Cyclo-Proginova is not used for contraception. If contraception is necessary, non-hormonal methods should be used (with the exception of calendar and temperature methods). If pregnancy is suspected, you should stop taking the pills until pregnancy can be ruled out (see section "Pregnancy and lactation"). If any of the following conditions or risk factors are present or worsening, the individual risk-benefit ratio of treatment should be assessed before starting or continuing HRT. Venous thromboembolism A number of controlled randomized and epidemiological studies have revealed an increased relative risk of developing venous thromboembolism (VTE) during HRT, i.e. deep vein thrombosis or pulmonary embolism. Therefore, when prescribing HRT to women with risk factors for VTE, the risk-benefit ratio of treatment should be carefully weighed and discussed with the patient. Risk factors for developing VTE include individual and family history (the presence of VTE in first-degree relatives at a relatively young age may indicate a genetic predisposition) and severe obesity. The risk of VTE also increases with age. The possible role of varicose veins in the development of VTE remains controversial. The risk of VTE may temporarily increase with prolonged immobilization, “major” elective and trauma surgeries, or major trauma. Depending on the cause or duration of immobilization, the question of the advisability of temporarily stopping HRT should be decided. Treatment should be stopped immediately if symptoms of thrombotic disorders appear or if their occurrence is suspected. Arterial thromboembolism In randomized controlled trials with long-term use of combined conjugated estrogens and medroxyprogesterone acetate, there was no evidence of a beneficial effect on the cardiovascular system. In large-scale clinical trials of this compound, a possible increased risk of coronary disease was identified in the first year of use. An increased risk of stroke was also found. To date, no long-term randomized controlled trials have been conducted with other HRT drugs to identify beneficial effects on cardiovascular morbidity and mortality. It is therefore unknown whether this increased risk applies to HRT products containing other types of estrogens and progestogens. Endometrial cancer Long-term estrogen monotherapy increases the risk of developing endometrial hyperplasia or carcinoma. Studies have confirmed that the addition of gestagens reduces the risk of endometrial hyperplasia and cancer. Breast cancer Clinical trials and observational studies have found an increase in the relative risk of breast cancer in women using HRT for several years. This may be due to earlier diagnosis, the biological effects of HRT, or a combination of both. The relative risk increases with the duration of treatment and may increase even more when estrogens are combined with progestogens. This increase is comparable to the increase in the risk of breast cancer in women with each year of delay in the onset of natural menopause, as well as with obesity and alcohol abuse. The increased risk gradually decreases to normal levels over the first few years after stopping HRT. According to research, breast cancer detected in women taking HRT is usually more differentiated than in women not taking it. HRT increases mammographic breast density, which in some cases may have a negative effect on X-ray detection of breast cancer. Liver tumor During the use of sex steroids, which include drugs for HRT, benign, and even more rarely, malignant liver tumors were observed in rare cases. In some cases, these tumors have resulted in life-threatening intra-abdominal bleeding. If there is pain in the upper abdomen, an enlarged liver, or signs of intra-abdominal bleeding, the differential diagnosis should take into account the possibility of a liver tumor. Gallstone disease Estrogens are known to increase the lithogenicity of bile. Some women are predisposed to developing gallstones when treated with estrogen. Other conditions Treatment should be stopped immediately if migraine-like or frequent and unusually severe headaches appear for the first time, as well as if other symptoms appear that are possible precursors of a thrombotic stroke of the brain. The relationship between HRT and the development of clinically significant arterial hypertension has not been established. A slight increase in blood pressure has been described in women taking HRT; clinically significant increases are rare. However, in some cases, if persistent clinically significant arterial hypertension develops while taking HRT, discontinuation of HRT may be considered. For mild liver dysfunction, including various forms of hyperbilirubinemia, such as Dubin-Johnson syndrome or Rotor syndrome, medical supervision is necessary, and periodic liver function tests. If liver function tests worsen, HRT should be discontinued. If cholestatic jaundice or cholestatic pruritus recurs, first observed during pregnancy or prior treatment with sex steroid hormones, HRT should be stopped immediately. Special monitoring is required for women with moderately elevated triglyceride levels. In such cases, the use of HRT may cause a further increase in blood triglyceride levels, which increases the risk of acute pancreatitis. Although HRT may affect peripheral insulin resistance and glucose tolerance, there is usually no need to change the treatment regimen of diabetic patients when undergoing HRT. However, women with diabetes should be monitored when undergoing HRT. Some patients under the influence of HRT may develop undesirable manifestations of estrogen stimulation, such as abnormal uterine bleeding. Frequent or persistent pathological uterine bleeding during treatment is an indication for endometrial examination. If treatment for irregular menstrual cycles does not produce results, an examination should be performed to exclude an organic disease. Under the influence of estrogens, uterine fibroids can increase in size. In this case, treatment should be stopped. It is recommended to discontinue treatment if endometriosis relapses during HRT. If prolactinoma is suspected, this disease should be excluded before starting treatment. In some cases, chloasma may occur, especially in women with a history of chloasma during pregnancy. During HRT, women prone to chloasma should avoid prolonged exposure to the sun or ultraviolet radiation. The following conditions may occur or be aggravated by HRT. Although their relationship with HRT has not been proven, women with these conditions should be under medical supervision when undergoing HRT: epilepsy; benign breast tumor; bronchial asthma; migraine; porphyria; otosclerosis; systemic lupus erythematosus, chorea minor. Medical examination and counseling Before starting or resuming HRT, a woman is recommended to undergo a thorough general medical and gynecological examination (including examination of the mammary glands and cytological examination of cervical mucus) and exclude pregnancy. In addition, disorders of the blood coagulation system should be excluded. Control examinations should be carried out periodically. Impact on laboratory test results Taking sex steroids can affect biochemical indicators of liver, thyroid, adrenal and kidney function, plasma levels of transport proteins such as corticosteroid binding globulin and lipid/lipoprotein fractions, indicators of carbohydrate metabolism, coagulation and fibrinolysis. Influence on the ability to drive vehicles and operate machinery. No effect.
Storage conditions
List B.: At room temperature.
Best before date
60 months
ATX classification:
G Genitourinary system and sex hormones
G03 Sex hormones and modulators of the reproductive system
G03A Systemic hormonal contraceptives
G03AA Progestogens and estrogens (fixed combinations)
G03AA06 Norgestrel and estrogens
Cross-drug interactions
Before starting replacement HT, a woman must stop using hormonal contraceptives. If there is a need for protection, then you can use any suitable non-hormonal means.
It is necessary to take Cyclo-Proginova taking into account possible adverse reactions that develop when combined with other drugs:
A long course of drugs that induce liver enzymes (some antiepileptic and antimicrobial medications) can promote the clearance of sex hormones and thereby reduce their effectiveness. These drugs include barbiturates, Primidone, Rifampicin. Caution should be exercised if drugs containing oxarbazepine, Topiramate, or Griseofulvin are prescribed. Maximum rates of enzyme induction appear after 2-3 weeks of use, and may continue to act for another month after cessation of therapy.
In some patients, when Cyclo-Proginov is combined with drugs of the penicillin and tetracycline groups, there is a decrease in the concentration of estradiol.
Drugs used in replacement HT can change glucose tolerance. This must be taken into account in order to timely monitor the body’s response and adjust the dosage of antidiabetic oral drugs, as well as insulin.
Alcohol contained in alcoholic drinks or medications (tinctures, extracts) when combined with hormonal drugs increases the concentration of estradiol active in the body.
Drug for planning pregnancy
Cyclo-proginova refers to a hormonal drug and an artificial analogue of female sex hormones in the ovaries. The active substance - estradiol promotes:
- Improving blood circulation in the placental layer in women;
- Reducing the likelihood of miscarriage;
- Endometrial growth;
- Improving the attachment of the fertilized egg to the uterus;
- The production of natural hormones in the body for the rapid onset of a long-awaited pregnancy.
At the same time, it does not suppress ovulation, which increases the chances of conceiving and carrying a baby. In addition, it is possible to combine the drug with Utrozhestan, Actovegin, Duphaston as the best analogues in the presence of thin endometrium in women, leading only to the production of natural hormones and thickening of the endometrial layer. With the onset of pregnancy, a certain protection is created in the body of women against the possible development of various types of gynecological diseases. Estradiol is an artificial hormone that is important for women’s ovaries and helps not only replenish, but also eliminate the painful signs of menopause:
- Headaches;
- Nervous disorders;
- Pain when urinating.
After completing a treatment course with Cyclo-Proginova, sleep is normalized, women's general well-being improves, and the chances of conception increase when planning a pregnancy, when the period is constantly delayed due to an excessively thinned endometrium and the inability of the egg to attach to the uterus. The drug is indicated when planning pregnancy. According to reviews from women, conception occurs immediately after completing 1 course. If Proginova did not lead to conception and pregnancy, then most likely women have diseases in the pelvic organs, the endocrine system is disrupted, or sensitivity to the drug is reduced.
Side effects and overdose
Treatment with a hormonal drug can provoke a negative response from the body in the form of disruptions in internal systems:
- Reproductive system: change in the nature of MC (duration and intensity), breakthrough bleeding, spotting outside the MC schedule, dysmenorrhea, change in the structure of vaginal discharge, PMS-like state, tension and soreness of the mammary glands
- Gastrointestinal tract: flatulence, discomfort or pain, nausea with or without vomiting, exacerbation of intrahepatic cholestasis
- CVS: rapid heartbeat
- Skin: rash, itching, hyperpigmentation in certain areas of the skin (chloasma), acne, hair growth in uncharacteristic places
- Locomotor system: muscle cramps
- Central nervous system: headaches, migraines, vertigo, increased anxiety, depressive moods, convulsions, changes (increase/decrease) in body weight, loss of sexual desire, manifestations of individual allergies
- Eyes: decreased vision, unresponsiveness to wearing contact lenses
- Other effects: fatigue, swelling.
Consequences of taking large doses
So far there have been no recorded cases of dangerous side effects after overdoses of Cyclo-Proginov. Symptoms that may occur during drug intoxication include nausea, vomiting, and vaginal bleeding.
To eliminate negative consequences, symptomatic therapy is used.
Analogs
Thanks to pharmacists, today there are many products containing analogues of female hormones. Therefore, for each patient it is possible to select the most effective drug to eliminate various disorders of the reproductive system. But only a physician can determine which is best for replacement therapy.
Klimadinon
BIONORICA (Germany)
Average price: fl. (50 ml) – 418 rub., table. (60 pcs.) – 434 rub.
A remedy for reducing and eliminating the symptoms of menopause. The action of the drug is ensured by the properties of its main substance - compounds contained in the extract of the cohosh plant, or black cohosh.
The plant component has an estrogen-like effect, replenishing the lack of the body’s own hormones. Positively affects the state of the nervous system: reduces excitability, eliminates hot flashes (or softens the intensity), normalizes sleep.
The drug begins to act after several weeks of use (usually 2). The product is designed for long-term use, the course is determined individually for each patient.
The herbal remedy is produced in tablets and drops.
Tablets are taken 1-2 pieces daily, solution - twice a day, 30 drops in pure or diluted form.
Pros:
- Natural composition
- Eliminates sweating and fever.
Flaws:
- May cause exacerbation of chronic diseases
- Promotes weight gain.
Femoston
Abbott (Netherlands)
Average price: (28 tables) – 932 rubles.
Hormonal drugs are developed for use in replacement HT. Prescribed for disorders in the female body that arise as a result of menopause due to natural causes or surgical intervention. Also used in the prevention of osteoporosis in postmenopausal women.
The active ingredients are dydrogesterone and estradiol - artificially created substances that almost completely coincide in biological and pharmaceutical properties with endogenous female hormones.
The drug is presented as a set of two types of tablets (pink and pale yellow), designed for a 28-day course of use.
The dosage and duration of therapy is determined individually. The instructions prescribe to drink one tablet per day at one hour: first pink, and then yellowish.
Pros:
- Balanced set of hormones
- Helps with fractures.
Flaws:
- Side effects.