Write a review
Reviews: 0
Manufacturers: Warsaw Pharmaceutical Works Polfa
Active ingredients
- Molsidomin
Disease class
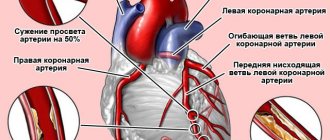
- Angina [angina pectoris]
- Congestive heart failure
Clinical and pharmacological group
- Not indicated. See instructions
Pharmacological action
- Antianginal
- Antiaggregation
- Vasodilating
Pharmacological group
- Nitrates and nitrate-like agents
Pharmacological properties of the drug Dilasid
Pharmacodynamics. Molsidomine is a derivative of sydnonimine. The active metabolite of molsidomine, linsidomine (SIN 1A), is a compound that reduces the tone of the smooth muscles of the vascular walls and has an antiplatelet effect. Relaxation of smooth muscles increases the volume of veins and the volume of the vascular bed, which leads to a decrease in venous return, thereby reducing the filling pressure of both ventricles. This reduces the load on the heart and improves the hemodynamic conditions of the coronary circulation. The dilation of arterial vessels causes a decrease in peripheral vascular resistance, as a result of which the load on the heart, myocardial tension are reduced and the myocardial oxygen demand is reduced. In addition, molsidomine reduces spasm of the coronary arteries and dilates their large branches. The antiplatelet effect of molsidomine is of clinical importance in the treatment of coronary artery disease. Unlike nitrates, molsidomine does not cause tachyphylaxis. Pharmacokinetics. After oral administration, molsidomine is absorbed from the gastrointestinal tract by approximately 90%. The onset of action appears 20 minutes after administration, the duration of action after a single dose is 4–6 hours. The maximum concentration in the blood is determined after approximately 30–60 minutes. Bioavailability is about 65%; it is bound to plasma proteins by 11%. Molsidomine is biotransformed in the liver enzymatically into the active metabolite, sydnonimine 1 (SIN-1), which is non-enzymatically transformed into morpholino-N-aminoacetonitrile (SIN 1A)-linsidomine. It is unknown whether molsidomine and its metabolites pass into breast milk. Molsidomine is mainly excreted in urine - 90-95% (2% unchanged) and feces - 3-4%. The overall clearance is 40–80 l/g, and SIN-1 is 170 l/g. The half-life of the drug is 1.6 hours, and in case of severe liver failure it increases (in case of liver cirrhosis - about 13.1 hours). The half-life of the linsidomine metabolite is 1–2 hours and increases in severe liver failure to 7.5 hours. The drug does not accumulate in the body.
Special instructions for the use of Dilasid
warnings and special measures for use. Molsidomine usually does not cause a significant decrease in blood pressure, however, patients with arterial hypotension, reduced blood volume and patients receiving other vasodilators should be careful. Elderly patients with hepatic or renal insufficiency should use lower doses. Considering that the drug contains lactose, it cannot be used in the treatment of patients with a rare form of congenital galactose intolerance, fructose intolerance, Lapp lactose deficiency or glucose-galactose malabsorption syndrome or sucrase-isomaltase deficiency. Use in children The drug is not used in pediatric practice. Use during pregnancy and lactation. Animal studies have not revealed a teratogenic effect of the drug. However, due to the lack of necessary studies in humans, the drug is contraindicated during pregnancy (especially in the first trimester). During breastfeeding, taking the drug is also contraindicated. If it is necessary to use molsidomine, breastfeeding should be discontinued during treatment. Effect on the ability to drive vehicles and operate machinery The drug usually does not affect the ability to drive vehicles and operate machinery. However, it is necessary to remember the possibility of orthostatic hypotension or dizziness in some cases.
Similar drugs:
- No-Spa Oral tablets
- Vinoxin-mv (VINCAMINUM) Oral tablets
- Liprazidum 10 Oral tablets
- Magnesium sulfate Powder for oral suspension
- Magnesium sulfate Substance-powder
- Niacin (Niacyne) Substance-powder
- Lisinopril Oral tablets
- Platyphyllin Oral tablets
- Parnavel Oral tablets
- Diroton Oral tablets
** The Drug Directory is intended for informational purposes only. For more complete information, please refer to the manufacturer's instructions. Do not self-medicate; Before starting to use Dilasid, you should consult a doctor. EUROLAB is not responsible for the consequences caused by the use of information posted on the portal. Any information on the site does not replace medical advice and cannot serve as a guarantee of the positive effect of the drug.
Are you interested in the drug Dilasid? Do you want to know more detailed information or do you need a doctor's examination? Or do you need an inspection? You can make an appointment with a doctor - the Euro lab is always at your service! The best doctors will examine you, advise you, provide the necessary assistance and make a diagnosis. You can also call a doctor at home . Euro lab clinic is open for you around the clock.
** Attention! The information presented in this medication guide is intended for medical professionals and should not be used as a basis for self-medication. The description of the drug Dilasid is provided for informational purposes and is not intended for prescribing treatment without the participation of a doctor. Patients need to consult a specialist!
If you are interested in any other drugs and medications, their descriptions and instructions for use, information about the composition and form of release, indications for use and side effects, methods of use, prices and reviews of drugs, or you have any other questions and suggestions - write to us, we will definitely try to help you.
Drug interactions with Dilasid
Molsidomine may enhance the effect of other vasodilators. When used simultaneously with peripheral vasodilators, calcium antagonists, ACE inhibitors, β-adrenergic receptor blockers, and diuretics, the hypotensive effect is potentiated. Simultaneous use with acetylsalicylic acid enhances the antiplatelet effect of the latter. The combined use of molsidomine and iloprost leads to a significant inhibition of platelet aggregation, therefore, if such a combination of drugs is necessary, appropriate studies should be carried out to assess the blood picture and the degree of platelet aggregation. When treating with the drug, sildenafil should not be used due to the possibility of irreversible arterial hypotension with dangerous consequences. Alcohol enhances the effect of the drug.
Dilaside
Active substance:
Molsidomine*
Pharmgroup:
Nitrates and nitrate-like agents
Analogs for the active substance:Corvaton Sydnopharm | Application area:Heberden's disease Angina pectoris Decompensated chronic heart failure Congestive circulatory failure Congestive heart failure with high afterload Congestive chronic heart failure Changes in liver function in heart failure Cardiomyopathy with severe chronic heart failure Compensated chronic heart failure Swelling due to circulatory failure Edema of cardiac origin Cardiac edema Edema syndrome in heart diseases Edema syndrome in congestive heart failure Edema syndrome in heart failure Edema syndrome in heart failure or cirrhosis of the liver Right ventricular failure Angina attack Recurrent angina Congestive heart failure Congestive heart failure Heart failure with low cardiac output Chronic heart failure Cardiac edema Spontaneous angina Stable angina Angina syndrome X Angina pectoris Angina (attack) Angina pectoris Angina at rest Angina pectoris progressive Mixed angina Angina pectoris spontaneous Angina pectoris stable Chronic decompensated heart failure Chronic congestive heart failure Chronic heart failure Chronic stable angina |
Overdose of the drug Dilasid
Symptoms: severe headache, arterial hypotension, tachycardia. In case of taking a dose significantly higher than the usual single dose, and if no more than 1 hour has passed after administration, it is necessary to rinse the stomach (provided that the patient is conscious). If necessary, carry out symptomatic treatment. In mild cases of overdose, it may be sufficient to place the patient in a horizontal position with the foot end of the bed raised upward. In case of severe overdose or an increase in the severity of symptoms, it is necessary to carry out infusion therapy, for example, physiological solution or other solutions necessary, in the opinion of the doctor, to replenish the bcc.