pharmachologic effect
Specific antagonist of angiotensin II type 1 receptors (AT1). Azilsartan medoxomil is a prodrug. It is quickly converted into the active azilsartan molecule, which selectively prevents the development of the effects of angiotensin II by blocking its binding to AT1 receptors in various tissues.
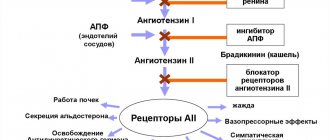
Angiotensin II is the primary vasoactive hormone of the RAAS with effects including vasoconstriction, cardiac stimulation, stimulation of aldosterone synthesis and release, and consequent renal sodium reabsorption. Blockade of AT1 receptors inhibits the negative regulatory response of angiotensin II to renin secretion, but the resulting increase in plasma renin activity and circulating angiotensin II levels does not suppress the antihypertensive effect of azilsartan.
The antihypertensive effect of azilsartan medoxomil develops during the first 2 weeks of use with the maximum therapeutic effect achieved after 4 weeks. A reduction in blood pressure after oral administration of a single dose is usually achieved within a few hours and persists for 24 hours.
Withdrawal syndrome after sudden cessation of treatment with long-term therapy (for 6 months) with Edarbi® was not observed.
The safety and effectiveness of the drug do not depend on the age of the patients, but greater sensitivity to lowering blood pressure in some elderly patients cannot be excluded. As with other angiotensin II receptor antagonists and ACE inhibitors, the antihypertensive effect is less pronounced in black patients (usually a population with low plasma renin activity). The simultaneous use of Edarbi® 40 mg and 80 mg with dihydropyridine blockers of slow calcium channels (amlodipine) or thiazide diuretics (chlorthalidone) leads to an additional reduction in blood pressure compared to therapy with antihypertensive drugs used in monotherapy.
Influence on repolarization processes:
The potential of Edarbi® to prolong the QT/QTc interval was assessed in healthy volunteers during a QT/QTc study. When using the drug Edarbi® at a dose of 320 mg, no increase in the QT/QTc interval was noted. QTc — corrected (relative to heart rate) value of the QT interval, relative value. Because The duration of the QT interval depends on the heart rate (lengthening as it slows down); to assess it, it must be corrected for heart rate. Prolongation of the QT interval reflects the heterogeneity of the processes of repolarization of the ventricular myocardium, and is regarded as an independent indicator indicating the possibility of fatal cardiac arrhythmias.
Effect of the drug on blood pressure
The blood pressure drug Edarbi Clo belongs to the group of specific AT1 receptor blockers and inhibits the proliferation of angiotensin. This hormone activates the following processes in the human body:
- Sodium absorption by the renal apparatus;
- Vasoconstriction;
- Activation of aldosterone release processes;
- Stimulation of cardiac muscle functions.
Due to the peculiarities of its composition, the medicine Edarbi lowers blood pressure. It is important that the tablets begin to act quickly, after 2-3 hours, and the therapeutic effect lasts throughout the day.
Edarbi begins to act quickly
Pharmacokinetics
Suction:
The estimated absolute bioavailability of azilsartan medoxomil when administered orally is approximately 60% based on plasma concentration profiles. Cmax of azilsartan in blood plasma is achieved on average within 1.5-3 hours after taking the drug orally. Food intake does not affect the bioavailability of azilsartan.
Distribution:
The pharmacokinetics of azilsartan medoxomil is dose proportional over a dose range of 20 mg to 320 mg after single or multiple oral administration. Vd of azilsartan is about 16 l. Azilsartan binds to plasma proteins (more than 99%), mainly to albumin. Plasma protein binding remains constant when azilsartan plasma concentrations are significantly higher than the range achieved when taken at recommended doses. Css of azilsartan is achieved within 5 days; its accumulation in the blood plasma does not occur with daily use once a day.
Animal studies with radioactive tracers have shown that the amount of azilsartan that penetrates the BBB is minimal.
Metabolism:
After oral administration during absorption from the gastrointestinal tract, azilsartan medoxomil is converted into the pharmacologically active metabolite azilsartan under the action of the enzyme carboxymethylenebutenolidase in the intestines and liver. Azilsartan is metabolized to two primary metabolites primarily in the liver. The main metabolite in blood plasma is formed by O-dealkylation and is designated as metabolite M-II, the minor metabolite is formed by decarboxylation and is designated as metabolite MI. The AUC values for these metabolites in humans are respectively 50% and less than 1% compared to azilsartan. MI and M-II do not affect the pharmacological activity of Edarbi®. The main enzyme responsible for the metabolism of azilsartan is the CYP2C9 isoenzyme.
Removal:
Azilsartan and its metabolites are excreted from the body, both through the intestines and the kidneys. Studies have shown that after oral administration of azilsartan medoxomil, about 55% (mainly as the metabolite MI) is found in the feces and about 42% (15% as azilsartan, 19% as the metabolite M-II) in the urine. T1/2 of azilsartan is about 11 hours, renal clearance is about 2.3 ml/min.
Categories
AllergistAnesthesiologist-resuscitatorVenereologistGastroenterologistHematologistGeneticGynecologistHomeopathDermatologistPediatric gynecologistPediatric neurologistPediatric urologistPediatric surgeonPediatric endocrinologistNutrologistImmunologistInfectious disease specialistCardiologistCosmetologistSpeech therapistElorologistMammologistMedical lawyerNarcologistNeurologistNeurosurgeon NephrologistOncologistOncourologistOrthopedist-traumatologistOphthalmologistPediatricianPlastic surgeonProctologistPsychiatristPsychologistPulmonologistRheumatologistRadiologistSexologist-AndrologistDentistTherapistUrologistPharmacistPhytotherapistPhlebologistSurgeonEndocrinologist
Pharmacokinetics in special groups of patients
The pharmacokinetics of azilsartan in children and adolescents under 18 years of age have not been studied.
The pharmacokinetics of azilsartan in young patients (18-45 years) and elderly patients (65-85 years) are not significantly different.
In patients with mild, moderate and severe renal impairment, AUC was increased by 30%, 25% and 95%, respectively. No increase in AUC (5%) was observed in patients with end-stage renal disease on hemodialysis. Clinical data on pharmacokinetics in patients with severe or end-stage renal failure are not available. Azilsartan is not removed from the systemic circulation by hemodialysis.
Use of Edarbi® for more than 5 days in patients with mild (Child-Pugh class A) or moderate (Child-Pugh class B) severity of liver failure leads to a slight increase in AUC (1.3-1.6 times, respectively). The pharmacokinetics of Edarbi® in patients with severe (class C on the Child-Pugh scale) degree of liver failure have not been studied.
The pharmacokinetics of azilsartan in men and women is not significantly different.
No dose adjustment is required depending on gender.
The pharmacokinetics of azilsartan do not differ significantly depending on the race of patients. No dose adjustment is required based on race.
Edarby Clo price
The cost of this medicine remains affordable for all regions of the Russian Federation. To orient yourself in prices, you should familiarize yourself with the price range in the Moscow region offered by different pharmacies. You can buy Edarby at approximately the following cost:
| Pharmacy on Tsvetnoy Boulevard | 645 rubles |
| Pharmacy IFC Novoslobodskaya | 707 rubles |
| Lighthouse on Krasnopresnenskaya | 649 rubles |
| ASNA - Mitino | 574 rubles |
Contraindications for use
- severe liver dysfunction (more than 9 points on the Child-Pugh scale) (no experience with use);
- pregnancy;
- simultaneous use of aliskiren in patients with diabetes mellitus;
- age under 18 years (efficacy and safety have not been established);
- hypersensitivity to the active substance and other components of the drug.
The drug should be used with caution in severe chronic heart failure (functional class IV according to the NYHA classification); severe renal failure; bilateral renal artery stenosis and stenosis of the artery of the only functioning kidney; ischemic cardiomyopathy; ischemic cerebrovascular diseases; condition after kidney transplantation; conditions accompanied by a decrease in blood volume (including vomiting, diarrhea), as well as in patients on a diet with limited salt; when used simultaneously with diuretics in high doses; primary hyperaldosteronism; hyperkalemia; stenosis of the aortic and mitral valves; hypertrophic obstructive cardiomyopathy (HOCM); in patients over 75 years of age.
Directions for use and dosage
The drug is taken orally 1 time per day, regardless of meals.
The recommended starting dose is 40 mg once a day. If additional blood pressure reduction is necessary, the dose of the drug can be increased to a maximum of 80 mg once a day. The maximum daily dose is 80 mg.
In case of inadequate blood pressure control when using Edarbi® as monotherapy, it can be used simultaneously with other antihypertensive drugs, including diuretics (chlorthalidone and hydrochlorothiazide) and dihydropyridine slow calcium channel blockers (amlodipine).
Edarbi® should be taken daily, without interruption. If treatment is stopped, the patient should inform the doctor.
If a dose is missed, the patient should take the next dose at the usual time. You should not take a double dose of Edarbi®.
No adjustment of the initial dose of Edarbi® is required in elderly patients. However, in patients over the age of 75 years, a dose of 20 mg can be considered as an initial dose (the risk of developing arterial hypotension increases).
No dosage adjustment is required in patients with mild to moderate renal impairment. There is no clinical experience with the use of Edarbi® in patients with severe renal impairment and end-stage renal failure, so the drug should be used with caution in this category of patients.
Due to limited experience with the use of Edarbi® in patients with mild to moderate liver dysfunction, it is recommended to begin treatment with a dose of 20 mg 1 time per day and carry it out under close supervision. The use of the drug in patients with severe liver dysfunction is not recommended due to lack of clinical experience.
Edarbi® should be prescribed to patients with decreased blood volume and/or hyponatremia (for example, patients with prolonged vomiting, diarrhea, or taking diuretics in large doses) only under strict medical supervision. It is recommended to start treatment with a dosage of 20 mg once a day.
Due to the lack of clinical experience, Edarbi® should be used with caution in patients with severe chronic heart failure (functional class IV according to the NYHA classification).
No dose adjustment is required in black patients. As with other angiotensin II (AT1) receptor antagonists and ACE inhibitors, black patients experience a smaller reduction in blood pressure compared to the rest of the population. In this regard, for adequate blood pressure control in patients of the Negroid race, it may be necessary to increase the dose of Edarbi® and complex therapy more often than in other patients.
Side effect
The frequency of adverse reactions was determined in accordance with WHO recommendations: very often (>1/10); often (>1/100, <1/10); uncommon (>1/1000, <1/100; rare (>1/10,000, <1/1000); very rare (<1/10,000), including isolated reports; unspecified frequency (frequency cannot be calculated from available data).
From the nervous system: often - dizziness.
From the cardiovascular system: infrequently - a pronounced decrease in blood pressure.
From the digestive system: often - diarrhea; infrequently - nausea.
From the skin and subcutaneous tissues: infrequently - rash, itching.
From the musculoskeletal system: infrequently - muscle spasms.
From laboratory and instrumental studies: often - increased CPK activity; uncommon - increased creatinine concentration, hyperuricemia.
Other: infrequently - increased fatigue, peripheral edema.
Description of individual adverse reactions:
With simultaneous use of the drug Edarbi® with chlorthalidone, the frequency of adverse reactions - a pronounced decrease in blood pressure and an increase in creatinine concentration - increases in frequency from infrequent to frequent.
With simultaneous use of Edarbi® with amlodipine, the frequency of an adverse reaction - peripheral edema - increases from infrequent to frequent, but is less common than with amlodipine monotherapy.
Angioedema, including swelling of the face, lips and periorbital edema, is rare.
As with the use of other angiotensin II receptor antagonists and ACE inhibitors, the simultaneous use of Edarbi® with diuretics (for example, chlorthalidone) leads to an increased incidence of increased creatinine concentrations. An increase in creatinine concentration with simultaneous use of Edarbi® with diuretics is associated with a more pronounced decrease in blood pressure compared to monotherapy with Edarbi®.
Most of these effects were short-lived or did not progress as long as patients continued therapy. After discontinuation of the drug, most cases of increase in creatinine concentration that did not go away during treatment were reversible. Creatinine concentrations returned to baseline values or values close to baseline in most patients.
When treated with Edarbi®, a slight increase in the concentration of uric acid in the blood serum (10.8 µmol/l) was observed compared to placebo (4.3 µmol/l).
As with the use of other RAAS inhibitors, when using Edarbi® as monotherapy, a slight decrease in hemoglobin and hematocrit was observed (on average, they decreased by about 3 g/l and 1 vol.%, respectively). If any of the side effects indicated in the instructions get worse, or the patient notices other side effects not listed in the instructions, you should inform your doctor.
What can be replaced?
Pharmacy chains offer the following analogues of Edarbi:
- Valaar;
- Sartavel;
- Lozap;
- Hypotene;
- Vasotens;
- Kozaar;
- Hyposard;
- Losartan;
- Angiakand;
- Nortivan;
- Blocktran;
- Cardiomin-Sanovel;
- Prosartran.
Experts categorically do not recommend self-medication and changing the drug Edarbi prescribed by the doctor to other medications that help lower blood pressure. Each of the analogues listed above is characterized by the presence of specific features, side effects, and restrictions on use. For this reason, if there is a need to replace Edarbi tablets, it is strongly recommended to seek advice from a qualified specialist who will help you choose the most effective and safe analogue.
Edarbi and Edarbi Clo tablets are an effective remedy that helps lower blood pressure and treat hypertension. Despite the small range of contraindications, this drug is allowed to be taken only after prior consultation with a specialist!
Use during pregnancy and breastfeeding
Pregnancy:
Animal studies have shown that azilsartan and M-II penetrate the placental barrier.
In patients planning pregnancy, treatment with alternative antihypertensive drugs with an established safety profile for pregnant women should be initiated. Immediately after confirmation of pregnancy, you should stop taking Edarbi® and, if necessary, begin a course of treatment with drugs approved for use during pregnancy.
Newborns whose mothers received therapy with Edarbi® may develop arterial hypotension, and therefore newborns should be under close medical supervision.
Lactation period:
There is no information on the ability of azilsartan and/or its metabolites to pass into breast milk. Animal studies revealed that azilsartan and M-II are excreted in the milk of activating rats.
Due to the lack of experience with the use of Edarbi® in women during breastfeeding, its use in this category of patients is not recommended. It is preferable to use drugs with the best studied safety profile, especially during the period of caring for a newborn or premature baby.
Fertility:
There are no data on the effects of Edarbi® on fertility in humans. Preclinical studies have shown no effect on male or female fertility in rats.
special instructions
Patients in whom vascular tone and renal function depend to a large extent on the activity of the RAAS (for example, in patients with severe chronic heart failure (NYHA functional class IV), severe renal failure or renal artery stenosis), treatment with drugs acting on RAAS, such as ACE inhibitors and angiotensin II receptor antagonists, is associated with the possibility of developing acute arterial hypotension, azotemia, oliguria or, rarely, acute renal failure. The possibility of developing the listed effects cannot be excluded when using Edarbi®.
A sharp decrease in blood pressure in patients with ischemic cardiomyopathy or ischemic cerebrovascular diseases can lead to the development of myocardial infarction or stroke.
There are no data on the use of Edarbi® in patients who have recently undergone kidney transplantation.
There are no data on clinical experience with the use of Edarbi® in patients with severe liver dysfunction, therefore the use of the drug in this category of patients is not recommended.
In patients with reduced blood volume and/or hyponatremia (as a result of vomiting, diarrhea, taking high doses of diuretics, or following a diet with limited sodium intake), clinically significant arterial hypotension may develop after initiation of therapy with Edarbi®. Hypovolemia should be corrected before starting treatment with Edarbi® or starting treatment with a dosage of 20 mg.
Patients with primary hyperaldosteronism are usually resistant to treatment with antihypertensive drugs that affect the RAAS. In this regard, Edarbi® is not recommended for use in such patients.
Clinical experience with other drugs that affect the RAAS shows that simultaneous administration of Edarbi® with potassium-sparing diuretics, potassium supplements or salt substitutes containing potassium, or other drugs that can increase potassium levels in the blood (for example, heparin) may lead to to hyperkalemia in patients with arterial hypertension. Elderly patients, patients with renal failure, diabetes mellitus and/or patients with other concomitant diseases increase the risk of developing hyperkalemia, which can be fatal. In such patients, it is recommended to monitor serum potassium levels.
When using Edarbi® in patients with aortic or mitral stenosis or hypertrophic obstructive cardiomyopathy, caution must be exercised.
As with the use of other angiotensin II receptor antagonists, simultaneous use of lithium and Edarbi® is not recommended.
Drug interactions
A reversible increase in serum lithium concentrations and toxicity were observed with simultaneous use of lithium preparations and ACE inhibitors and lithium preparations with angiotensin II receptor antagonists. Therefore, the simultaneous use of azilsartan medoxomil in combination with lithium preparations is not recommended. If it is necessary to use this combination, regular monitoring of lithium levels in the blood serum is recommended.
With the simultaneous use of angiotensin II antagonists and NSAIDs (for example, selective COX-2 inhibitors, acetylsalicylic acid (more than 3 g / day) and non-selective NSAIDs), the antihypertensive effect may be weakened. With simultaneous use of angiotensin II antagonists and NSAIDs, the risk of renal dysfunction and an increase in serum potassium may increase. Therefore, at the beginning of treatment, patients are advised to regularly take sufficient fluids and monitor kidney function.
Concomitant use of potassium-sparing diuretics, potassium supplements, salt substitutes containing potassium and other drugs (for example, heparin) with azilsartan medoxomil may lead to an increase in serum potassium levels. Patients should monitor serum potassium levels during combination therapy.
Dual blockade of the RAAS with angiotensin II receptor antagonists, ACE inhibitors or aliskiren is associated with an increased risk of hypotension, hyperkalemia, and renal dysfunction (including acute renal failure) compared with monotherapy.
No pharmacokinetic interactions were observed with simultaneous use of azilsartan medoxomil or azilsartan with amlodipine, antacids (magnesium and aluminum hydroxide), chlorthalidone, digoxin, fluconazole, glibenclamide, ketoconazole, metformin and warfarin.
Azilsartan medoxomil is converted into the pharmacologically active metabolite azilsartan during absorption from the gastrointestinal tract under the action of the enzyme carboxymethylenebutenolidase in the intestines and liver. In vitro studies have shown that interactions based on enzyme inhibition are unlikely.
The antihypertensive effect of azilsartan medoxomil therapy can be enhanced when used in combination with other antihypertensive agents, including diuretics (chlorthalidone and hydrochlorothiazide) and dihydropyridine slow calcium channel blockers (amlodipine).
What is Edarbi clo
Edarbi clo contains the active ingredient azilsartan medoxomil potassium. Doctors can prescribe the drug for hypertension and general instability of blood pressure. When using Edarbi, there is no clinical effect at the beginning of administration. Additional doses of diuretics can lower blood pressure better and more effectively. During diuretic therapy, the patient should monitor blood pressure. It must be remembered that exceeding the dosage has a detrimental effect on the cardiovascular system.