| Prescription drug | |
| Release form | Solution for subcutaneous administration, 250 mcg/0.5 ml. 0.5 ml of solution containing 250 mcg (6500 IU) choriogonadotropin alpha. In a colorless glass syringe with an injection needle, closed with a protective cap with a rubber gasket. The syringe is packed in plastic |
| Manufacturer | Merck Serono S.p.A. (Italy) |
| Active substance | Choriogonadotropin alpha |
To learn more
Consultations are conducted by professional doctors who have significant experience in solving infertility problems.
Release form and composition
| Lyophilisate for the preparation of solution for subcutaneous administration | 1 syringe |
| Choriogonadotropin alpha | 250 mcg (6500 IU) |
| Excipients: | |
| mannitol | 27.3 mg |
Ovitrel contains human chorionic gonadotropin alpha. Its dose is 250 mcg, which corresponds to 6500 IU. Available in the form of a solution, which is contained in filled syringes.
Ovitrel is a lyophilisate for subcutaneous administration. It appears as a white or almost white powder. The resulting solution is transparent. It may be colorless or slightly yellowish.
Ovitrel is a drug of recombinant human chorionic gonadotropin. But it has the same biological effects as hCG obtained from the urine of pregnant women.
Which is better to choose: Ovitrel or Pregnil?
These drugs are similar in action
. Both the first and second are widely practiced by doctors.
Pregnyl is stronger. You can learn more about the drug Pregnil in this article.
Under its influence, fewer follicles are formed, but they are of higher quality.
Have you used the medicine and was it effective for you?
pharmachologic effect
Ovitrel has the same range of physiological and biochemical effects as natural human chorionic gonadotropin. Its effects:
- It has high luteinizing activity, surpassing LH (luteinizing hormone) in this indicator.
- Keeps the corpus luteum functionally active. This is a temporary organ that forms at the site of a burst follicle. It produces hormones, particularly progesterone. Thanks to Ovitrel, the corpus luteum does not disappear after 2 weeks, but performs its function during the first 3 months of pregnancy. Until the placenta takes over the function of producing hormones.
- Ovitrel stimulates the production of estrogen. To a lesser extent, it enhances the production of weak androgens in women. These hormones are produced by the follicular apparatus of the ovaries.
- Ovitrel slightly enhances the formation of adrenal hormones. In women, it causes functional hyperplasia of the organ. As a result, the production of glucocorticoids increases, which promotes adaptation to stress.
- During pregnancy, Ovitrel supports the function of the placenta and improves its blood circulation. The hCG hormone increases the number of chorionic villi.
- In men, Ovitrel stimulates spermatogenesis and enhances the production of androgens.
Medicinal properties
The therapeutic effect of Ovitrel is achieved thanks to the main component - choriogonadotropin alpha (CG alpha). The substance was created using genetic engineering; in its structure, the amino acids are given in the same order as in the human hCG hormone.
The main task of the substance in the female body is to restore oocyte meiosis, normalize ovulation, form the corpus luteum with the subsequent production of hormones that ensure the correct formation and gestation of the fetus. HCG alpha promotes the development of a reaction comparable to a sharp rise in the level of LH, which is responsible for ovulation.
Ovitrel is in demand to stimulate the full maturation of follicles, subsequent luteinization (after additional use of drugs that activate their growth). Clinical comparisons showed that Ovitrel 250 mcg had the same effect that was observed after administration of 5-10 thousand IU of human hCG. So far, scientists have not recorded cases of the formation of antibodies to the drug. The results of repeated use of the drug were studied exclusively in men. Use in women usually requires one therapeutic course.
After administration, the active substance of Ovitrel passes into the intercellular space, the half-life of distribution takes about 4.5 hours. Metabolism is no different from endogenous hCG. In any case, there is no data yet to refute the observation.
The half-life of the active component of the drug from the body is on average 30 hours, and bioavailability is almost 40%. When comparing the characteristics of the lyophilisate and the finished solution, it became clear that they were completely similar.
Indications for use
The instructions for use of the drug Ovitrel indicate two indications for its use:
- In the IVF protocol. Ovitrel is used in combination with other drugs. For the maturation of eggs in the ovaries, gonadotropins are prescribed. Then, after a few days of using them, when the follicles reach the required size (as determined by ultrasound), the doctor administers Ovitrel. It is used as an ovulation trigger. The main purpose of using the drug is to prevent the follicles from rupturing prematurely. Because premature ovulation will not allow you to obtain eggs for artificial insemination.
- In the treatment of infertility due to anovulation or oligoovulation. That is, when a woman, due to endocrine reasons, does not ovulate (the maturation of the follicle and the release of an egg) at all or in most cycles. Oligoovulation is a condition when there is no ovulation for several months, but not more than six months. If there is no ovulation for 6 or more months, this is anovulation. Both pathologies are essentially the same. The terms reflect only the degree of hormonal imbalance. Ovitrel is used for the same purpose. After drug induction of ovulation, the purpose of which is usually the maturation of one follicle, hCG is prescribed. After the Ovitrel injection, sexual intercourse is recommended one day later.
Analogues substitutes
The drug Ovitrel has its own analogues, the effect of which is the same:
- Horagon;
- Ecostimulin;
- Chorionic gonadotropin;
- Decayed;
- Profasi;
- Gonakor.
Photo gallery of analogues:
Choragon Human chorionic gonadotropin
Decayed
Profasi
Gonakor
Contraindications for use
Ovitrel is contraindicated in women with the following diseases:
Tumors of the hypothalamic-pituitary system. Since these are endocrine structures, they produce hormones. Tumors can grow from hormone-producing cells. Often, against the background of cancer, the production of gonadotropins increases, so their use in the form of drugs is not recommended.
Ovarian tumors or cysts (except polycystic ovary syndrome). The use of hormonal drugs can stimulate their growth. It also provokes an increase in the size of the ovaries. As a result, the risk of cyst rupture or ovarian torsion increases.
Breast, ovarian, endometrial cancer. These three cancers are united by the fact that they are all hormone-dependent in most cases. The more sex hormones in the blood, the faster the malignant tumor grows and spreads. Therefore, for cancer of the corresponding localization, a woman often receives drugs that block hormones or their receptors. This slows down the growth of the tumor. If Ovitrel is used, the amount of estrogen in the blood increases. This adversely affects the course of these diseases.
Bleeding from the genital tract of unknown origin. It is taken into account that such bleeding may be caused by a hitherto undetected malignant tumor of the uterus. Therefore, first the woman is examined. She is undergoing hysteroscopy. If there are no tumor processes and bleeding has been eliminated, infertility treatment can be carried out, including the use of Ovitrel.
Recent ectopic pregnancy. The drug is not prescribed for 3 months after its cessation. The body must recover to be ready for the next stimulation.
Thromboembolism. A history of thromboembolism is a reason to refuse stimulation or reduce the dose of drugs as much as possible. Because an increase in the level of sex hormones in the blood leads to increased coagulation and an increased risk of blood clots.
Insufficiency of ovarian function. The use of ovulation stimulation and Ovitrel in such a situation seems futile. If the ovaries can no longer produce follicles containing eggs, then no medications will help stimulate their function. The doctor evaluates how appropriate it is to treat infertility in this way based on a number of indicators. The number of antral follicles in the first days of the cycle, the level of FSH and AMH are taken into account.
Malformations of the reproductive organs. In this case, pregnancy is generally contraindicated for the woman. Because its outcome will most likely be unfavorable.
Large uterine fibroids. Incompatible with pregnancy. And since Ovitrel is used exclusively for the purpose of its onset, the use of the drug in the presence of a large tumor is contraindicated.
Postmenopause. There is no point in using Ovitrel. In postmenopause, your own eggs no longer mature in the ovaries.
The drug is considered contraindicated during pregnancy and lactation. However, the danger of its use during gestation seems unlikely. After all, a pregnant woman’s body already produces a huge amount of hCG. Injecting it into a patient when she is unaware of her pregnancy will not significantly increase the concentration of this hormone in the blood. However, there is no practical sense in prescribing the drug to pregnant women. After all, it is used in the treatment of infertility.
Mode of application
Ovitrel is administered subcutaneously. In this case, its bioavailability is about 40%. One syringe is intended for one use. Even if not the entire dose has been administered, the syringe cannot be reused.
The order of introduction is as follows:
- Wash your hands.
- Prepare 2 cotton swabs, alcohol, Ovitrel.
- Clean the injection site with alcohol. This is the anterior wall of the abdomen. Can also be injected into the thigh. Gather the skin into a fold with your fingers. Directing the needle at an angle of 45 degrees, an injection should be made. The drug is administered slowly. Then the needle is removed and the injection site is wiped.
Injecting Ovitrel into a vein should be avoided. Therefore, during the injection process, it is advisable to check whether the needle has entered the vessel. To do this, pull the piston a little. If blood enters the syringe, continuing the injection is not recommended. It is worth removing the needle. Then inject again in another place using another syringe.
- After the injection, the syringe must be disposed of.
Dosage
For use after hormonal stimulation of ovulation, including in the IVF protocol, Ovitrel is administered once, at a dose of 6500 IU. This is the contents of 1 syringe. It is administered 1-2 days after discontinuation of FSH and LH drugs. In this case, hCG allows for optimal follicular development.
Subscribe to our newsletter
Subscription benefits
I accept the terms of the User Agreement and consent to the processing of my personal data
Ovitrel is used for anovulation or oligoovulation. It is used in conjunction with other drugs that induce ovulatory processes. The drug is administered 24 or 48 hours after stimulation ceases and the follicles reach their optimal size. The day after the injection, fertilizing sexual intercourse is recommended.
Side effects
A study of the effect of Ovitrel on the female body revealed that the undesirable effects are dose-dependent. Most often there are:
- Gastrointestinal tract: nausea, vomiting, abdominal pain, diarrhea
- Reproductive system: OHSS, breast tenderness, ectopic pregnancy
- NS: depressive states, increased nervousness, anxiety
- Immunity: allergic manifestations
- Skin: minor dermal reactions in the form of temporary rashes.
In addition, thromboembolic phenomena are possible after a hormonal drug.
special instructions
Before starting treatment, patients are examined to determine the causes of infertility. All women undergo blood tests for hormones. The use of ovulation induction regimens using the drug Ovitrel is indicated only in cases where restoration of natural fertility is impossible. But sometimes it is possible to restore it. For example, in such cases:
- adrenal insufficiency - hormonal drugs are prescribed for replacement therapy;
- hypothyroidism - sodium levothyroxine is used to compensate for impaired production of thyroid hormones;
- hyperprolactinemia - relieved by drugs that inhibit the formation of prolactin in the pituitary gland (bromocriptine or other drugs of this pharmacological group).
It should be taken into account that the administration of Ovitrel enhances the function of the thyroid gland. This primarily affects those patients who suffer from uncompensated thyrotoxicosis. Their production of thyroxine and triiodothyronine may increase even more, which will result in aggravation of the clinical symptoms of endocrine disorders.
Using Ovitrel may cause false positive pregnancy tests. This medicine contains hCG. It is this hormone that is determined in urine during tests. Its increased concentration in the blood can persist for up to 10 days after administration. Accordingly, pregnancy tests carried out during this period in case of a positive result cannot be considered unambiguously valid. If the test result is negative, the result is most likely correct unless there are other reasons for distorting the data obtained.
Reviews from women
Angelina, 36 years old : “Ovitrel solution was injected during IVF. I went through a whole course of therapy. At first I was afraid that nothing would work out and that I would have to repeat the course again. Thank God I got pregnant the first time. The pregnancy was multiple. Now I’m a happy mother of twins and grateful to this drug.”
Svetlana, 35 years old : “My husband and I really want to have our own children. I was on Ovitrel solution several times, and other drugs were also used. But so far to no avail.”
Risk of OHSS
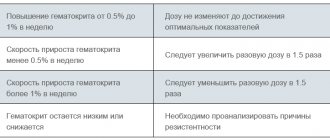
Ovitrel is used in superovulation stimulation schemes. It should be taken into account that with this method of infertility treatment there is a risk of ovarian hyperstimulation syndrome. To reduce it as much as possible, the doctor carefully assesses the woman’s condition before stimulation begins. It takes into account the level of FSH, estradiol, the number of antral follicles, the woman’s age, and the presence of gynecological diseases. Based on this, a stimulation scheme is selected. In subsequent cycles, the doctor takes into account the experience of previous stimulations and, based on the response of the ovaries, adjusts the doses of hormones.
Drug dosages can also be adjusted during the stimulation process itself. The doctor monitors the reaction of the ovaries by changes in the level of hormones in the blood and the number of growing follicles in the ovaries. If necessary, the dosage of medications is reduced.
Hyperstimulation syndrome, if it occurs, can be early or late. Early is easier, it occurs before pregnancy. Late develops in the case of successful infertility treatment, when pregnancy occurs. Its symptoms are usually more severe. Hyperstimulation syndrome is manifested by enlarged ovaries, edema, and decreased diuresis. Sometimes it leads to the accumulation of fluid in the abdominal cavity, and may be complicated by ovarian torsion or thromboembolism.
It has been established that during drug treatment of anovulation, the risk of developing OHSS increases significantly if:
- estradiol level above 1500 pg/ml;
- more than 3 follicles with a diameter of over 14 mm grow.
Indicators that are considered risk factors in the case of IVF:
- estradiol above 3000 pg/ml;
- more than 18 follicles over 11 mm in diameter.
The pregnancy rate is high if 2 or more embryos are transferred into the uterus at the same time.
Cancellation of Ovitrel allows you to avoid ovarian hyperstimulation syndrome. Therefore, if significant risk factors are detected, the doctor may stop treatment. It carries over to the next cycle. In this case, smaller doses of gonadotropins are used for subsequent stimulation. This makes the treatment process safer.
Other risks
Multiple pregnancy. With artificial insemination, the risk of multiple pregnancy is high if 2 or more embryos are transferred into the uterus at the same time. If Ovitrel is used in the process of ovulation induction in women with anovulatory disorders, the goal of treatment is the maturation of one dominant follicle in each cycle. If 2 or more follicles mature, there is a risk of multiple pregnancy. Further tactics are agreed upon with the patient. If she is willing to accept this risk, Ovitrel is administered and sexual intercourse is recommended. If a woman is afraid of multiple births, then she should stop stimulation, refuse to administer hCG and avoid intimate life for the next few days - until the end of the ovulatory phase.
Miscarriage. The frequency of miscarriages and frozen pregnancies among women receiving treatment with Ovitrel is higher than the average values in the population. However, the reason for this is not the drug itself, but the health status of the patients. Healthy women do not use Ovitrel; they can become pregnant on their own. And if they cannot, the cause is often endocrine disorders. They lead to undeveloped pregnancy.
Ectopic pregnancy. The risk of developing tubal pregnancy when using IVF is higher than the average in the population. However, the administration of Ovitrel does not increase it. The higher risk is due to the fact that women with fallopian tube disease are often treated for infertility.
Congenital developmental anomalies. The risk of congenital genetic and chromosomal diseases in a child conceived using the drug Ovitrel is higher than the average in the population. However, the drug itself does not play any role in this. The reason is the poor reproductive health of parents who are being treated for infertility. Additionally, the average age of patients who become pregnant through IVF or drug ovulation induction is significantly higher than those who become pregnant naturally. Many patients turn to reproductive medicine clinics when they are 35-40 years old, and sometimes older. With age, the genetic material of germ cells inevitably deteriorates. The risk of genetic defects can be reduced to almost zero if preimplantation genetic diagnosis is included in the IVF program.
Overdose
There is no official information about the possibility of overdose with the drug. Reviews available about the drug Ovitrel indicate the possibility of ovarian hyperstimulation. In this case, cavities begin to form in them, and excess fluid accumulates in the abdomen. Fortunately, the situation is not dangerous. If such an overdose occurs, you must stop using the drug, abstain from sexual activity for 4-5 days and inform your doctor about the consequences of using the medication.
Drugs from the same pharmacological group
Gonal-f
Luveris
Lucrine depot
Orgalutran
Pergonal
Decayed
Puregon - instructions for use
Horagon