Release form and composition
Leucostim is produced in the form of a solution for subcutaneous and intravenous administration:
- 150 mcg: in 1 ml bottles, 5 bottles in a cardboard box;
- 300 mcg: in bottles of 1 or 1.6 ml, 5 bottles in a cardboard pack, or 1 or 5 bottles in contour plastic packages, 1 pack in a cardboard box;
- In glass syringes with needles of 0.5 or 1 ml, 1 or 5 syringes in a cardboard box;
- 600 mcg: in syringes of 0.8 ml, 1 or 5 syringes in blister packs, 1 pack in a cardboard box).
1 ml contains the active substance: filgrastim - 150, 300 or 600 mcg.
Analogs
Level 4 ATC code matches:
Neupogen
Neupomax
Granocyte
Thymogen
Taktivin
Granogen , Grasalva , Leucita , Neupogen , Mielastra , Neipomax , Neurostim , Filergim .
Indications for use
- Neutropenia (including in patients receiving cytotoxic drugs in the treatment of non-myeloid malignancies);
- Persistent neutropenia in patients with an advanced stage of HIV infection (with an absolute neutrophil count of 1000 cells/μl or less);
- Neutropenia (hereditary, idiopathic or recurrent with a neutrophil count less than or equal to 500 cells/μl) and severe or recurrent infections (history) within the last 12 months.
Leucostim is also used to reduce the duration of neutropenia and its clinical consequences in patients who are preparing for bone marrow transplantation and to mobilize peripheral stem cells (including after myelosuppressive therapy).
Reviews about Leukostim
There are few reviews from patients who have taken this drug. For example, there are reviews of the use of this drug for cyclic neutropenia and immune neutropenia in children . In these severe diseases, the effect of the drug was short-lived.
neutropenia associated with chemotherapy of neoplasms are more common . Thus, the drug was prescribed to children at a dose of 5 mcg/kg per day and was well tolerated. There are results of clinical studies, the purpose of which was to compare the tolerability and effectiveness of Leucostim and Neupogen . Conclusions were drawn: the time frame for eliminating neutropenia when using these drugs does not differ, and they have identical tolerability and effectiveness indicators.
Directions for use and dosage
Leucostim is administered subcutaneously (preferably) or intravenously. If intravenous administration of the drug is necessary, the required amount of Leucostim with a 5% dextrose solution should be injected from a syringe into a vial or plastic container, then infused for 30 minutes. The drug cannot be diluted with 0.9% sodium chloride solution. Also, filgrastim should not be diluted to a final concentration of less than 2 mcg/ml (0.2 million IU/ml).
The use of the drug is not recommended less than 24 hours before the start and earlier than 24 hours after the end of chemotherapy.
Doses and route of administration are determined by the specific clinical situation.
For neutropenia, Leucostim should be administered intravenously or subcutaneously once a day at a dose of 5 mcg per 1 kg of patient body weight after a course of cytotoxic chemotherapy.
In patients receiving cytotoxic chemotherapy, a transient increase in the number of neutrophils is usually observed 1-2 days after the start of therapy. To assess the effectiveness of treatment, it is advisable to count the number of neutrophils in the peripheral blood daily. Therapy is recommended until the number of neutrophils reaches normal values. Leucostim can be discontinued once the absolute neutrophil count exceeds 2.0×109/L. If necessary, the duration of the treatment course can be up to 12 days.
After myeloablative chemotherapy followed by bone marrow transplantation, Leucostim should be administered intravenously or subcutaneously at the rate of 10 mcg per 1 kg of body weight. The first dose should be administered no earlier than 24 hours after cytotoxic chemotherapy; for bone marrow transplantation, no later than 24 hours after bone marrow infusion. The daily dose of Leukostim is adjusted after the moment of maximum decrease in the number of neutrophils has passed. In cases where the content of neutrophils in the peripheral blood is higher than 1.0x109/l for three days in a row, the dose of Leucostim is reduced by 2 times (to 5 mcg per 1 kg of body weight). Then, if for three consecutive days the absolute number of neutrophils exceeds 1.0x109/l, Leucostim is discontinued. If the absolute number of neutrophils decreases during therapy below 1.0x109/l, the dose is again increased to 10 mcg per 1 kg of body weight.
When using Leukostim for the purpose of mobilizing hematopoietic stem cells, subcutaneous administration is recommended in a daily dose of 5 mcg (in patients after myelosuppressive chemotherapy) or 10 mcg per 1 kg of patient weight (in the absence of chemotherapy) for 5-7 consecutive days (the number of injections is determined by the effectiveness of separation and the rate of increase in the number of leukocytes in peripheral blood). The day before the first separation (on the fourth day of Leukostim administration) and during the following days before the day of the last separation, the number of neutrophils and leukocytes in the patient’s peripheral blood should be assessed. Cytapheresis is carried out in cases of an increase in the number of leukocytes to 5x109/l of peripheral blood, starting from the fifth day of Leucostim administration. After each separation, the number of CD34+ cells and nucleated cells in the sample intended for cryopreservation must be counted. The administration of Leukostim is stopped when the number of cryopreserved CD34+ cells sufficient for transplantation is reached (not less than 2 × 106 per 1 kg of patient weight).
The safety and effectiveness of Leukostim in healthy donors under 16 and over 60 years of age has not been studied.
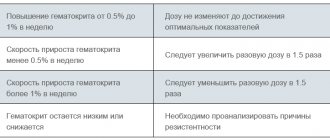
In the treatment of severe chronic neutropenia (SCN), Leucostim should be administered subcutaneously daily. The use of the drug is stopped after the number of neutrophils stably exceeds 1.5x109/l (for congenital neutropenia - at a dose of 12 mcg per 1 kg of patient weight per day subcutaneously for one or more injections; for periodic or idiopathic neutropenia - 5 mcg per 1 kg weight per day). To maintain this level of neutrophils, the minimum effective dose is determined. This requires daily continuous administration of the drug. Depending on the patient’s response, after 7-14 days of therapy, the initial dose can be increased or decreased by 2 times. In the future, every 7-14 days it is necessary to adjust the dose to maintain the number of neutrophils within the range of 1.5-10x109/l.
When treating neutropenia associated with HIV infection, Leucostim is administered subcutaneously once at an initial dose of 1-4 mcg per 1 kg of body weight per day until the number of neutrophils normalizes (more than 2x109/l). As a rule, normalization of the number of neutrophils occurs after 2 days. If therapy is ineffective, the dose is escalated to 5 mcg per 1 kg of body weight per day once subcutaneously. After achieving a therapeutic effect, they switch to maintenance therapy - 2-3 times a week, 1-4 mcg per 1 kg of body weight per day. In the future, individual dose adjustment is possible.
Pharmacological properties
Pharmacodynamics
The active substance of Leukostim, filgrastim, is a highly purified non-glycosylated protein consisting of 175 amino acids. It is produced by a strain of Escherichia coli, into the genome of which the G-CSF (human granulocyte colony-stimulating factor) gene has been introduced using genetic engineering methods. Human G-CSF is a glycoprotein that regulates the formation of functionally active neutrophils and their release from the bone marrow into the blood.
As a result of the use of Leucostim, the number of neutrophils in the peripheral blood increases significantly within 24 hours after administration, with a slight increase in the number of monocytes. In SCN (severe chronic neutropenia), filgrastim may lead to a slight increase in the number of circulating basophils and eosinophils. Some of these patients may exhibit basophilia or eosinophilia even before treatment begins.
Filgrastim dose-dependently increases the number of neutrophils with normal/increased phagocytic and chemotactic activity. After the end of therapy, the number of neutrophils in the peripheral blood decreases by 50% in 1–2 days; the indicator returns to normal levels over the next 1–7 days.
The use of Leukostim can significantly reduce the frequency, duration and severity of neutropenia and febrile neutropenia, while reducing the need and duration of hospital treatment in patients receiving chemotherapy with cytostatics or myeloablative treatment followed by bone marrow transplantation.
Patients receiving Leucostim and cytotoxic chemotherapy require lower doses of antibiotics compared to patients receiving cytotoxic chemotherapy without filgrastim.
The use of the drug significantly reduces the duration of febrile neutropenia, the need for antibiotic therapy and hospitalization after induction chemotherapy in patients with acute myeloid leukemia. At the same time, filgrastim has no effect on the frequency of fever and infectious complications.
The use of Leukostim both independently and after chemotherapy helps to mobilize the release of hematopoietic stem cells into the peripheral bloodstream. Allogeneic or autologous transplantation of PSCC (peripheral blood stem cells) is performed after therapy with large doses of cytostatics, or instead of/in addition to bone marrow transplantation. PSCC transplantation may also be indicated after myelosuppressive cytotoxic treatment. The use of PSCC mobilized with filgrastim can accelerate the recovery of hematopoiesis, reduce the risk of hemorrhagic complications and the need for platelet transfusion after myeloablative or myelosuppressive therapy.
The safety/efficacy profile of Leukostim in adults and children receiving cytotoxic chemotherapy does not differ.
In children and adults with SCN (severe congenital, periodic, idiopathic neutropenia), filgrastim consistently increases the number of neutrophils in the peripheral blood and reduces the incidence of infectious complications.
When Leukostim is prescribed to patients with HIV infection, it becomes possible to maintain normal neutrophil levels and comply with the recommended doses of antiretroviral and/or other myelosuppressive therapy. There are no signs of increased HIV replication during filgrastim therapy.
Like other hematopoietic growth factors, G-CSF has a stimulating effect on human endothelial cells.
The carcinogenic properties of filgrastim have not been studied. Filgrastim does not cause mutations in the bacterial genome, regardless of the presence of the enzyme system necessary for drug metabolism.
It has been established that certain malignant cells have receptors for G-CSF on their surface. It cannot be excluded that filgrastim may serve as a growth factor for various types of tumors.
When conducting studies on rats of both sexes, using filgrastim in doses up to 500 mcg/kg, no effect on fertility or pregnancy was found.
In studies on rabbits and rats, filgrastim had no teratogenic effect. An increased rate of miscarriages was observed in rabbits, but no fetal developmental anomalies were identified.
Pharmacokinetics
Filgrastim is rapidly absorbed after subcutaneous administration and reaches its maximum concentration in the blood serum within 2–8 hours. The half-life after intravenous or subcutaneous administration is usually in the range of 2–4 hours. Clearance and half-life are determined by dose and neutrophil count. It can be assumed that the linear nature of clearance and pharmacokinetic processes predominates.
Absolute bioavailability after subcutaneous administration of 375 and 750 mcg is 62% and 72%, respectively. The concentration of filgrastim after stopping its administration decreases to endogenous values within 24 hours.
In patients with cancer and healthy volunteers before chemotherapy, repeated administration of filgrastim revealed a decrease in its plasma concentration. The increase in clearance of the substance in this case is dose-dependent, presumably the degree of this increase depends on the degree of neutrophilia in recipients. This is consistent with information about an increase in neutrophil-dependent clearance with an increase in the neutrophil pool. In patients who receive filgrastim after chemotherapy, the plasma concentration of the substance remains at the same level until the start of hematopoiesis recovery.
With intravenous and subcutaneous administration of filgrastim, a positive linear relationship is observed between dose and serum concentration. After subcutaneous administration of therapeutic doses, the concentration of filgrastim exceeds 10 ng/ml for 8–16 hours. The volume of distribution is 150 ml/kg.
With long-term administration of filgrastim (course up to 28 days) after autologous bone marrow transplantation, accumulation and changes in the half-life are not observed.
Regardless of the route of administration, the elimination of filgrastim proceeds according to the rules of first-order kinetics. The half-life is 3.5 hours, clearance is 0.6 ml/min/kg.
The pharmacokinetics of filgrastim in children after chemotherapy is similar to that in adults who receive the same doses of the drug based on body weight. This allows us to conclude that the pharmacokinetic processes of Leukostim are independent of age.
In end-stage renal disease, there is a trend towards increased systemic exposure to filgrastim compared to healthy volunteers and patients with creatinine clearance in the range of 30-60 ml/min.
Side effects
The use of Leucostim may be accompanied by muscle and bone pain, as well as pain at the injection site.
In 7.5% of patients, moderate or mild musculoskeletal pain was noted, which did not require drug correction or was relieved by non-steroidal anti-inflammatory drugs. No severe pain was noted during therapy.
In rare cases, delayed-type hypersensitivity reactions may develop at the site of drug administration, accompanied by the appearance of edema and erythema (if they develop, the use of Leucostim should be discontinued).
In some cases, when using the drug, hepatomegaly, fatigue, headache, diarrhea, and urinary disorders (mainly moderate or mild dysuria) were observed. There were isolated reports of a transient decrease in blood pressure (no treatment required). It is also possible to develop a reversible, dose-dependent and usually weak or moderate increase in the concentrations of alkaline phosphatase, lactate dehydrogenase, serum uric acid and gamma-glutamyltransferase, and a decrease in the number of platelets in the peripheral blood.
Rarely, skin rash, vascular thrombosis, vasculitis, and enlarged spleen may occur in patients with an initially non-enlarged spleen. Infiltrates may appear in the lungs with the development of respiratory distress syndrome in adults. Such phenomena occurred more often after the use of chemotherapy regimens that included bleomycin; their connection with taking Leukostim has not been established. Very rarely, cases of hematuria and proteinuria have been observed after using the drug.
It is extremely rare that rheumatoid arthritis is aggravated when using the drug; with long-term use, splenic rupture, anemia, thrombocytopenia, as well as skin vasculitis in patients with severe chronic neutropenia may occur.
special instructions
Treatment with Leukostim should only be carried out under the supervision of an oncologist or hematologist with experience in the use of such drugs. Before prescribing it, causes of transient neutropenia such as viral infections should be excluded.
Particular attention must be paid to the diagnosis of severe chronic neutropenia to differentiate it from other hematological diseases such as myeloid leukemia, myelodysplasia and aplastic anemia.
In chronic myeloid leukemia and myelodysplastic syndrome, the safety and effectiveness of filgrastim have not been established. For patients with these diseases and with precancerous lesions of the myeloid lineage of hematopoiesis, Leucostim is not recommended, because Some tumor cells may carry a receptor for granulocyte colony-stimulating factor. For this reason, special attention should be paid to the differential diagnosis between blast crisis of chronic myeloid leukemia and acute myeloid leukemia.
During therapy, it is necessary to constantly monitor the size of the spleen (by palpation of the abdomen). According to research data, when the dose of Leucostim is reduced, the enlargement of the spleen stops or, at least, slows down.
A small number (about 3%) of patients with Kostmann's syndrome treated with filgrastim experienced leukemia and myelodysplastic syndrome, natural complications of this disease, the relationship of which with the use of the drug has not been established. If these complications develop, Leucostim should be discontinued.
In rare cases (less than 5%), hyperleukocytosis (increase in white blood cell count above 100x109/L) has been observed in patients undergoing treatment with filgrastim, so the white blood cell count should be determined regularly. If they increase to more than 50×109/l, Leucostim should be discontinued. If the drug is used to mobilize hematopoietic stem cells, it should be discontinued if the leukocyte count increases to more than 70 × 109/L.
In approximately 12% of patients with initially normal cytogenetics, abnormalities, including monosomy 7, were detected on repeat testing. If cytogenetic abnormalities are detected in patients with severe congenital neutropenia, the benefits and possible risks of continuing therapy should be weighed. Every 12 months it is necessary to conduct cytogenetic and morphological studies of the bone marrow.
It is important to consider that Leucostim does not prevent anemia and thrombocytopenia, which are often a consequence of the use of chemotherapy drugs in high doses. Therefore, during treatment after chemotherapy, the number of platelets and red blood cells, as well as the level of hemoglobin, should be regularly determined.
Impact on the ability to drive vehicles and complex mechanisms
Considering the mechanism of pharmacological action of filgrastim, its effect on the speed of psychomotor reactions and the ability to concentrate seems extremely unlikely.
Drug interactions
It is necessary to observe an interval of 24 hours (before or after) when using Leucostim simultaneously with myelosuppressive drugs.
The safety and effectiveness of administering filgrastim on the same day as cytotoxic chemotherapy has not been established.
Pharmaceutically, Leucostim is not compatible with 0.9% sodium chloride solution.
When administered concomitantly with filgrastim, 5-fluorouracil may increase the severity of neutropenia.
When using the drug to mobilize hematopoietic stem cells after chemotherapy, it must be taken into account that when prescribing cytostatics such as carboplatin, carmustine (BCNU) and melphalan for a long time, the effectiveness of mobilization may be reduced.
Contraindications
Absolute:
- Kostmann's syndrome (severe congenital neutropenia) with cytogenetic disorders;
- Use for the purpose of increasing doses of cytotoxic chemotherapy drugs exceeding the recommended ones;
- Simultaneous use with cytotoxic chemotherapy and radiation therapy;
- Lactation period;
- Individual intolerance to the components of the drug.
Relative (Leukostim is prescribed with caution):
- Sickle cell anemia;
- Pathology of bone tissue (including osteoporosis);
- Secondary acute myeloid leukemia (due to limited safety/efficacy data);
- Use in combination with high-dose chemotherapy;
- Pregnancy.
Safety and efficacy have not been assessed:
- Children under 16 years of age after myelosuppressive or myeloablative therapy followed by autologous transfusion of PSCC;
- Newborns with severe chronic neutropenia.