Pharmacological action of Epocrine
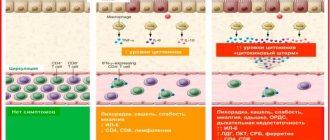
Epocrine is a recombinant erythropoietin, whose action is aimed at stimulating erythropoiesis.
It is able to activate mitosis and maturation of red blood cells from progenitor cells. Recombinant epoetin alpha is synthesized in mammalian cells that contain a gene capable of encoding human erythropoietin.
The composition of mammalian epoetin, as well as its immunological and biological properties, are similar to human erythropoietin.
When epoetin alfa is introduced into the human body, the level of hematocrit and hemoglobin increases, blood supply to tissues and heart function improves.
The greatest effectiveness of the drug is observed in those types of anemia that are caused by chronic renal failure.
In rare cases, long-term use of epoetin in the treatment of anemia can lead to the formation of neutralizing antibodies to human erythropoietin, and partial red cell aplasia sometimes develops.
Epocrine 10000 IU No. 10 ampoules
pharmachologic effect
Erythropoiesis stimulator, recombinant human erythropoietin, glycoprotein.
Activates mitosis and maturation of red blood cells from precursor cells of the erythrocyte series. Recombinant epoetin alfa is synthesized in mammalian cells into which the gene encoding human erythropoietin is integrated. In its composition, biological and immunological properties, epoetin alfa is identical to natural human erythropoietin. The administration of epoetin alfa leads to an increase in hemoglobin and hematocrit levels, improvement in blood supply to tissues and heart function.
The most pronounced effect of the use of epoetin alfa is observed in anemia caused by chronic renal failure.
In very rare cases, with long-term use of epoetin alfa for the treatment of anemic conditions, the formation of neutralizing antibodies to erythropoietin may be observed with or without the development of partial red cell aplasia.
Indications
- anemia in patients with chronic renal failure (including those on hemodialysis);
- prevention and treatment of anemia in patients with solid tumors resulting from antitumor therapy;
- prevention and treatment of anemia caused by the use of zidovudine in HIV-infected patients (AIDS);
- prevention and treatment of anemia in patients with multiple myeloma, low-grade non-Hodgkin's lymphoma, chronic lymphocytic leukemia, and in patients with rheumatoid arthritis;
- prevention and treatment of anemia in premature infants born with low body weight (up to 1500 g);
- to reduce the volume of blood transfused during major surgical interventions and acute blood loss.
Dosage regimen
When treating anemia in patients with chronic renal failure, Epocrin® is administered subcutaneously or intravenously; for patients on hemodialysis - through an arteriovenous shunt at the end of the dialysis session. If the route of administration is changed, the drug is administered at the same dose, then the dose is adjusted if necessary (with subcutaneous administration of the drug Epokrin®, to achieve the same therapeutic effect, a dose of 20-30% less is required than with intravenous administration). Treatment with Epocrine includes 2 stages.
1. Correction stage. With subcutaneous administration of the drug Epocrine®, the initial dose is 30 IU/kg 3 times a week. With intravenous administration of the drug Epocrine®, the initial dose is 50 IU/kg. The correction period lasts until the optimal level of hemoglobin (100-120 g/l in adults and 95-110 g/l in children) and hematocrit (30-35%) is achieved. These indicators must be monitored weekly.
The following situations are possible:
The effectiveness of therapy depends on a correctly selected individual treatment regimen.
2. Maintenance therapy stage. To maintain the hematocrit at a level of 30-35%, the dose of Epokrin® used at the correction stage should be reduced by 1.5 times. Then the maintenance dose of Epocrine® is selected individually, taking into account the dynamics of hematocrit and hemoglobin. After stabilization of hematological parameters, it is possible to switch to administration of the drug Epokrin® once every 1-2 weeks.
When preventing and treating anemia in patients with solid tumors, it is recommended to determine the level of endogenous erythropoietin before using the drug. When the concentration of serum erythropoietin is less than 200 IU/ml, the initial dose of Epokrin® for intravenous administration is 150 IU/kg. With subcutaneous administration, the initial dose of Epocrine® can be reduced to 100 IU/kg. If there is no response, the dose may be increased to 300 IU/kg. Further increase in dose seems inappropriate. It is not recommended to prescribe Epokrin® to patients with a serum endogenous erythropoietin content of more than 200 IU/ml.
For the prevention and treatment of anemia caused by the use of zidovudine in patients with HIV infection, intravenous administration of the drug Epokrin® at a dose of 100-150 IU/kg 3 times a week is effective provided that the level of serum endogenous erythropoietin is less than 500 IU/ ml, and the dose of zidovudine is less than 4.2 g per week. With subcutaneous administration, the dose of Epocrine® can be reduced by 1.5 times.
The advisability of using the drug Epokrin® for the prevention and treatment of anemia in patients with multiple myeloma, low-grade non-Hodgkin's lymphoma, chronic lymphocytic leukemia is due to inadequate synthesis of endogenous erythropoietin against the background of the development of anemia. If the hemoglobin content is less than 100 g/l and serum erythropoietin is below 100 IU/ml, Epocrine® is administered subcutaneously at an initial dose of 100 IU/kg 3 times a week. Laboratory monitoring of hemodynamic parameters is carried out weekly. If necessary, the dose of Epocrine® is adjusted upward or downward every 3-4 weeks. If, upon reaching a weekly dose of 600 IU/kg, no increase in hemoglobin levels is observed, Epocrine® should be discontinued, because its further use is ineffective.
The feasibility of using the drug Epokrin® for the prevention and treatment of anemia in patients with rheumatoid arthritis is due to the fact that in this disease there is suppression of the synthesis of endogenous erythropoietin under the influence of an increased concentration of proinflammatory cytokines. Epocrine® is administered subcutaneously at a dose of 50-75 IU/kg 3 times a week. If the hemoglobin level increases by less than 10 g/l after 4 weeks of treatment, the dose of Epokrin® is increased to 150-200 IU/kg 3 times a week. Further increase in dose seems inappropriate.
For the prevention and treatment of anemia in premature infants born with low body weight, Epocrine® is administered subcutaneously at a dose of 200 IU/kg 3 times a week, starting from the 6th day of life until the target hemoglobin and hematocrit values are achieved, but not more than 6 weeks .
To prevent anemia during extensive surgery and acute blood loss, Epocrine® is administered intravenously or subcutaneously 3 times a week at a dose of 100-150 IU/kg until hematocrit and hemoglobin content normalize.
Side effect
- Influenza-like syndrome: in some cases, at the beginning of therapy - dizziness, drowsiness, fever, headache, myalgia, arthralgia.
- From the cardiovascular system: dose-dependent arterial hypertension, worsening of arterial hypertension are possible (more often in patients with uremia); in some cases - a hypertensive crisis, a sharp increase in blood pressure with symptoms of encephalopathy (headache, confusion) and generalized tonic-clonic convulsions.
- From the side of metabolism: possible decrease in serum ferritin concentration, decrease in serum levels of iron metabolism; In patients with uremia, hyperkalemia and hyperphosphatemia are possible.
- Allergic reactions: possible - mild or moderate skin rash, urticaria, itching, angioedema, eczema.
- Local reactions: possible hyperemia, burning, mild or moderate pain at the injection site (more often occurs with subcutaneous administration).
- Other: thrombocytosis; in some cases - shunt thrombosis (in patients on hemodialysis, with a tendency to arterial hypotension or with an aneurysm, stenosis); symptoms associated with breathing problems or unstable blood pressure; very rarely - immune reactions (induction of antibody formation with or without the development of partial red cell aplasia), exacerbation of porphyria.
Contraindications for use
- partial red cell aplasia after previous therapy with any erythropoietin;
- uncontrolled arterial hypertension;
- inability to carry out adequate anticoagulant therapy;
- period within 1 month after myocardial infarction;
- unstable angina;
- increased risk of deep vein thrombosis and thromboembolism as part of the pre-deposit blood collection program before surgery;
- porphyria;
- hypersensitivity to the components of the drug.
The drug should be prescribed with caution to patients with thrombosis (history), with malignant neoplasms, with sickle cell anemia, in patients with moderate anemia without iron deficiency, in patients with refractory anemia, epilepsy and chronic liver failure.
Before using the medicine, we recommend that you consult your doctor online
Use during pregnancy and breastfeeding
Since there is insufficient experience with the use of epoetin alfa during pregnancy and lactation in humans, Epocrine should be prescribed only in cases where the expected benefit of therapy for the mother outweighs the potential risk to the fetus or infant.
When using epoetin alfa in women of reproductive age with anemia due to chronic renal failure, menstruation may resume. The patient should be warned about the possibility of pregnancy and the need to use reliable methods of contraception before starting therapy.
Before using the medicine, we recommend that you consult your doctor online
Use for liver dysfunction
The drug should be prescribed with caution to patients with chronic liver failure.
special instructions
The possibility of an increase in blood pressure at the beginning of therapy should be borne in mind. Considering the possible more pronounced effect of the drug Epokrin®, its dose should not exceed the dose of recombinant erythropoietin, which was used in the previous course of treatment. During the first two weeks, the dose is not changed while assessing the dose/response relationship. After this, the dose can be reduced or increased according to the above scheme.
During treatment, it is necessary to monitor blood pressure weekly, perform a general blood test, including determination of hematocrit, platelets and ferritin. In patients with uremia undergoing hemodialysis, due to an increase in hematocrit, it is often necessary to increase the dose of heparin; in addition, timely prevention of thrombosis and early revision of the shunt are necessary.
In the pre- and postoperative period, hemoglobin should be monitored more often if its initial level was less than 140 g/l.
It must be borne in mind that epoetin alfa does not replace blood transfusion, but reduces the volume and frequency of its use. In patients with controlled hypertension or thrombotic complications, an increase in the dose of antihypertensive and/or anticoagulant drugs may be required. If a hypertensive crisis develops, emergency treatment is carried out; treatment with epoetin alfa in such cases should be temporarily discontinued.
When prescribing Epocrine® to patients with liver failure, its metabolism may slow down and erythropoiesis may significantly increase. The safety of epoetin alfa in this category of patients has not been established.
We cannot exclude the possibility that epoetin alfa may influence the growth of certain types of tumors, incl. bone marrow tumors.
The possibility that a preoperative increase in hemoglobin levels may predispose to the development of thrombotic complications should be considered. Before starting treatment, possible causes of an inadequate reaction to the drug should be excluded (deficiency of iron, folic acid, cyanocobalamin, severe poisoning with aluminum ions, concomitant infections, inflammatory processes and injuries, hidden blood loss, hemolysis, bone marrow fibrosis of various etiologies) and, if necessary, adjust treatment.
In most patients with uremia, cancer and HIV-infected patients, plasma ferritin levels decrease simultaneously with an increase in hematocrit. Ferritin levels must be determined throughout the course of treatment. If it is less than 100 ng/ml, replacement therapy with iron preparations for oral administration is recommended at a rate of 200-300 mg/day (for children - 100-200 mg/day). For premature infants, oral iron supplements at a dose of 2 mg/day should be prescribed as early as possible. Patients who donate autologous blood and are in the pre- or postoperative period should also receive adequate therapy with iron supplements in a dose of up to 200 mg/day.
In patients with uremia, correction of anemia with epoetin alfa may result in improved appetite and increased absorption of potassium and protein. In this regard, periodic adjustment of hemodialysis parameters may be required to maintain the levels of urea, creatinine and potassium within normal limits. Serum electrolyte levels should also be monitored in these patients.
Impact on the ability to drive vehicles and operate machinery
During the treatment period, until the optimal maintenance dose is established, patients with uremia should avoid engaging in potentially hazardous activities that require increased concentration and high speed of psychomotor reactions.
Overdose
Symptoms: possible increased side effects.
Treatment: in case of increased blood pressure, antihypertensive drugs are prescribed; if the latter are ineffective, Epocrine® should be discontinued. If hemoglobin and hematocrit levels are high, bloodletting is indicated.
Drug interactions
With simultaneous use of the drug Epokrin® with cyclosporine, the binding of the latter to erythrocytes increases (adjustment of the dose of cyclosporine may be required).
Based on the currently available experience in the clinical use of the drug Epokrin®, no evidence of its pharmacological incompatibility with other drugs has been identified. However, to avoid possible incompatibility or decreased activity, Epocrine® should not be mixed with solutions of other drugs.
Storage conditions and periods
The drug should be stored out of the reach of children at a temperature of 2° to 8°C. Shelf life: 2 years.
Indications for use of Epocrine
Epocrine is recommended for use in the following cases:
- for anemia in patients with chronic renal failure (including those on hemodialysis);
- as a prophylaxis and for the treatment of anemia that occurs after long-term antitumor therapy;
- for the prevention and treatment of anemia caused by the use of zidovudine in patients infected with HIV;
- as a prophylaxis and in the treatment of anemia in patients diagnosed with myeloma, with non-Hodgkin lymphomas, the degree of malignancy of which is defined as “low”, with rheumatoid arthritis and chronic lymphocytic leukemia;
- for the prevention and treatment of anemia in premature infants born weighing less than 1500 g;
- in order to reduce the volume of blood transfused during acute blood loss and surgical interventions.
Dosing of the drug
When treating anemia in patients suffering from kidney failure, Epocrine is administered intravenously. And for those patients who are on hemodialysis, this is done through an arteriovenous shunt. If the method of administration is changed, the drug is used in the same dosage, and then it can be adjusted if necessary. Treatment with the drug "Epocrine" includes the following two stages:
- The first stage is the correction stage. When administering the drug "Epocrine", the initial dosage is 30 IU per kilogram three times a week. When administered intravenously, the initial dosage is 50 IU/kg. The correction period continues until the patient reaches the most optimal hemoglobin level (from 100 to 120 grams per liter in adults and from 95 to 110 in small patients). The hematocrit should be between thirty and thirty-five percent. These indicators need to be monitored weekly.
- Maintenance treatment phase. To maintain the hematocrit at the level of thirty-five percent, the dosage of the drug used at the adjustment stage should subsequently be reduced by one and a half times. Then the maintenance dosage of Epocrine is selected individually, taking into account the dynamics of hemoglobin and hematocrit. After stabilization of the hematological indicator, a quick transition to using the drug once every two weeks is possible. Analogues of Epocrine 1000 IU can be purchased at any pharmacy.
Contraindications
Epocrine has a number of contraindications; the instructions for Epocrine recommend that you familiarize yourself with them before starting to take the medicine. Epocrine should not be used in the following cases:
- for partial red cell aplasia if therapy with erythropoietin has already been carried out;
- with uncontrolled arterial hypertension;
- if it is impossible to carry out adequate anticoagulant treatment;
- during the period within a month after myocardial infarction;
- with unstable angina;
- with an increased risk of thromboembolism and deep vein thrombosis;
- with porphyria;
- with hypersensitivity to any of the components of Epocrine.
Epocrine is prescribed with caution to the following groups of patients:
- patients with various malignant neoplasms;
- patients with thrombosis (including a history);
- patients with sickle cell anemia;
- patients with epilepsy;
- patients with chronic liver failure;
- patients with moderate anemia without iron deficiency.
Cheap analogues of Epocrine
The drug currently has the following cheap analogues:
- The drug "Aeprin" will cost consumers 1,500 rubles. This medicine is taken for anemia, and also for the treatment of kidney failure and peritoneal dialysis. This analogue of Epocrine is prescribed to HIV-infected patients, as well as for non-myeloid tumors, including against the background of cytostatic treatment.
- The medicine "Eralfon" costs about 1,600 rubles. This cheap analogue of Epocrine is also used if the patient has anemia. Otherwise, the indications coincide with the previous analogue "Aeprin".
- The analogue of Epokrin, Eprex, costs about 1,300 rubles. It is prescribed for anemia, peritoneal dialysis, non-myeloid tumors, and so on.
Instructions for use of Epocrine
In the treatment of anemia in patients with chronic renal failure, Epocrine is administered subcutaneously or intravenously. Changing the route of administration requires dose adjustment only if necessary (it should be noted that to achieve the same therapeutic effect with subcutaneous administration, 20-30% less drug is required than with intravenous administration). The optimal hemoglobin (HB) content in the blood of patients with anemia in chronic renal failure: in adults - from 100 to 120 g/l, in children - from 95 to 110 g/l.
Treatment with Epocrine is divided into two stages:
- correction stage, in which the initial dose of Epocrine for subcutaneous administration is 30 IU/kg three times a week (for intravenous administration - 50 IU/kg with the same frequency). The stage lasts until the optimal level of HB concentration is reached. Monitoring should be done weekly. In this case, the following situations are possible: a) if the hematocrit increases from 0.5 to 1% within a week, the dose is not changed; b) hematocrit increases by less than 0.5% per week - increase the dose by 1.5 rubles; c) the growth rate is more than 1% over the same period - reduce the dose by 1.5 rubles; d) the hematocrit does not change or decreases - an analysis of the causes of resistance is needed;
- stage of maintenance therapy. The hematocrit should be kept at 30-35%; for this, the dose of Epocrine used at the correction stage is reduced by 1.5 times. After which the maintenance dose of Epocrine is selected taking into account the individual dynamics of hemoglobin and hematocrit. Stabilization of hematological parameters allows the administration of Epocrine to be prescribed once every 1-2 weeks.
The initial and final dosage of Epocrine is determined in accordance with the disease, condition and age of the patient:
- Before starting treatment and prevention of anemia with Epocrine in patients with solid tumors, the level of endogenous erythropoietin should be determined. If it is less than 200 IU/ml, the initial dose for intravenous administration should be 150 IU/kg (for subcutaneous administration - 100 IU/kg). The dose may be increased to 300 IU/kg (maximum) if there is no response;
- in the treatment and as a prophylaxis of anemia associated with the use of zidovudine in HIV-positive patients, intravenous administration of Epocrine in the amount of 100-150 IU/kg is recommended only if the dose of zidovudine is not more than 4.2 g per week and the level of endogenous erythropoietin does not exceed 500 IU/ml;
- prevention and treatment of anemia in patients with rheumatoid arthritis is carried out with Epocrine subcutaneously at a dosage of 50-75 IU/kg three times a week. Then, if the HB content increases by less than 10 g/l, the dose can be increased to 150-200 IU/kg (maximum), the frequency remains the same;
- prevention and treatment of anemia in children born with low body weight: dosage is 200 IU/kg body weight three times a week. It is necessary to start from the 6th day of life until normal hematocrit and hemoglobin levels are achieved, but no longer than 6 weeks;
- Prevention of anemia during acute blood loss and operations is carried out by intravenous or subcutaneous administration of Epocrine in an amount of 100 to 150 IU/kg. When hemolytic parameters are normalized, therapy is canceled.
Epocrine
Active substance:
Epoetin alfa*
Pharmgroup:
Hematopoiesis stimulants
Average price in pharmacies
| Name | Manufacturer | average price |
| Epocrine 10000IU/1ml n10 amp d/v/v s.c. | FSUE "State Research Institute of OCB" FMBA of Russia | 16706.00 |
| Epocrine 1000IU/1ml n10 amp d/v/v s.c. | FSUE "State Research Institute of OCB" FMBA of Russia | 2721.00 |
| Epocrine 2000IU/1ml n10 amp solution d/v/v s.c. | FSUE "State Research Institute of OCB" FMBA of Russia | 4434.00 |
| Epocrine 4000IU/1ml 3000IU/0.75ml n10 amp solution IV s.c. | FSUE "State Research Institute of OCB" FMBA of Russia | 5102.00 |
Analogs for the active substance:Aeprin Rapoetin-SP Eprex Eralfon |
Side effects of Epocrine
You should carefully read the instructions for Epocrine, since the use of the drug can cause adverse reactions, including:
- drowsiness, dizziness, headache, arthralgia, myalgia;
- worsening of arterial hypertension, hypertensive crisis;
- hyperkalemia, hyperphosphatemia, decreased serum ferritin levels;
- hyperemia, burning, pain at the injection site;
- itching, urticaria, eczema;
- thrombocytosis, exacerbation of porphyria, respiratory and blood pressure disorders.
"Rapoetin-SP"
It is considered an analogue of Epocrine 2000 IU. The active ingredient is epoetin alfa.
Indicated for anemia: chronic renal failure, HIV infection during treatment with Zidovudine, hemodialysis, cytostatic chemotherapy, in premature newborns; preparing patients for surgery that may result in massive blood loss.
Contraindicated in case of hypersensitivity, uncontrolled arterial hypertension, iron deficiency, pregnancy, breastfeeding.
The Epocrine analogue 2000 IU often provokes hypertension, headache, fatigue, asthenia, dizziness, arthralgia, chest pain, nausea, vomiting, diarrhea, edema, thrombophilia, convulsions, skin reaction at the injection site.
Should not be used in combination with solutions of other products. Hematocrit should be regularly monitored to prevent loss of efficacy and development of resistance.
"Ipidacrine": analogue or not?
Is "Epocrine" an analogue of "Ipidacrine"? The drugs in question have different active substances, so they are only distant substitutes for each other. In “Epocrine” the active substance is a component called epoetin alfa, and in “Ipidacrine” it is the same name as the drug ipidacrine.
The drug "Ipidacrine" acts as a reversible cholinesterase inhibitor; it can prevent the hydrolysis of acetylcholine, prolonging its effect. This medication blocks membrane potassium channels, which directly promotes their depolarization. The drug in question stimulates synaptic transmission in nerve and muscle endings, and in addition, enhances the effect of acetylcholine on smooth muscles.
This medication restores nerve and muscle transmission along with excitation in the nervous system. During its use in patients, the tone increases along with the contractility of the smooth muscles of the organs, the secretion of the salivary glands increases along with the contractile activity of the myometrium and the tone of the skeletal muscles. The drug "Ipidacrine" produces a stimulating effect on the nervous system in combination with a separate manifestation of a sedative effect. The use of this medicine helps improve memory and learning. Drugs containing an active substance called ipidacrine include “Axamon” along with “Amiridin”, “Ipigrix” and “Neuromidin”.
Reviews of "Epocrine" and analogues
People speak differently about the drug "Epocrine" in their comments. It is often noted that this drug effectively helps with anemia.
Patients, as well as doctors, praise drug analogues for their effectiveness and assistance in the treatment of low-grade non-Hodgkin lymphoma and chronic lymphocytic leukemia. Among other things, comments about these drugs indicate the advisability of their use in acute blood loss.
As for negative reviews, most often we are talking about the high cost of Epocrine and similar products, and in addition, about the frequent occurrence of certain adverse reactions. For example, people during treatment complain of headaches, cramps and flushing.
We reviewed the description of Epocrine and analogues.