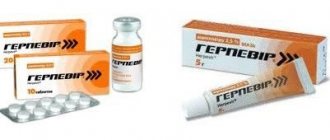
pharmachologic effect
Antifungal drug, allylamine. Has a wide spectrum of antifungal action.
Active against dermatophytes: Trichophyton rubrum, Trichophyton mentagrophytes, Trichophyton tonsurans, Trichophyton verrucosum, Trichophyton violaceum, Microsporum canis, Epidermophyton floccosum. In low concentrations, it has a fungicidal effect on dermatophytes, molds (including Aspergillus, Cladosporium, Scopulariopsis brevicaulis) and some dimorphic fungi. Depending on the species, the drug has a fungicidal or fungistatic effect on yeast fungi of the genus Candida (mainly Candida albicans) and their mycelial forms.
Thermikon® disrupts the early stage of biosynthesis of the main component of the cell membrane of the fungus, ergosterol, by inhibiting the enzyme squalene epoxidase. This leads to ergosterol deficiency and intracellular accumulation of squalene, which causes the death of the fungal cell. Terbinafine acts by inhibiting the enzyme squalene epoxidase located on the cell membrane of the fungus.
When taken orally, it is not effective in the treatment of pityriasis versicolor caused by Pityrosporum orbiculare (Malassezia furfur).
Thermikon cream
TERMIKON® cream is a modern external antifungal drug for the treatment and prevention of fungal skin lesions.
Advantages of the active substance of TERMIKON cream:
- wide spectrum of antifungal activity – action against various types of fungi that affect human skin (dermatophytes, molds and yeasts)1
- multiple superiority in the degree of antifungal activity over other antifungal molecules, incl. clotrimazole, miconazole, econazole, etc.2
- the ability to have a “fungicidal” effect on fungal cells1, i.e. not just contribute to a slowdown in growth and loss of their ability to reproduce, but cause their immediate death
- creating therapeutic concentrations in the stratum corneum of the skin within the first 4 hours from the moment of the first application and maintaining them as such after the cessation of local treatment (when applied once a day for 7 days) for an additional 7 days3,4
- the ability to maintain a protective and preventive effect after a course of treatment lasting up to 3 months3.5
- the presence, in addition to the direct antifungal effect, also has anti-inflammatory6 and antibacterial7 activity, which is especially important since a fungal infection can often be accompanied by an inflammatory process and the addition of a secondary bacterial infection; the presence of additional properties makes it possible to treat skin mycoses with erythema and swelling without additional prescription of topical corticosteroids and the use of antimicrobrons;
- Possibility of one-time use per day for most fungal skin lesions1, ensuring ease of use for consumers
- the possibility of conducting short treatment courses lasting 1 week1, which is 4 times shorter than a course of clotrimazole therapy8
Advantages of the TERMIKON cream release form:
for primary purpose:
- fungal infections of the feet, accompanied by dryness, peeling and cracks
- fungal infections of smooth body skin, limited in area
- prevention of fungal infection, incl. in persons with increased dryness of the skin of the feet caused by impaired blood supply to the lower extremities
by release form:
- The delicate, velvety, homogeneous consistency of the cream makes it easy to apply to the skin
- unlike fatty ointments, the cream base does not form a continuous film on the surface of the skin, creating a “greenhouse effect” and disrupting the process of natural respiration of the skin; the cream form allows the drug to be used on affected areas with signs of inflammation
- The hydrophilic base of the cream promotes more complete and rapid absorption of the drug into the skin compared to fat-based ointments
- The cream base contains components that provide a softening and moisturizing effect on dry and rough skin areas due to fungal infection.
by packaging:
- light protection
- resistance to external mechanical influence
- compactness
Indications for use of TERMIKON cream:
prevention and treatment of fungal skin infections, including:
- mycoses of the feet (“fungus” of the foot);
- inguinal athlete's foot ("fungus" of the folds);
- fungal infections of smooth body skin;
- infections caused by yeast-like fungi of the genus Candida (candidiasis), in particular diaper rash;
- pityriasis versicolor (pityriasis versicolor).
Contraindications to the use of TERMIKON cream:
- hypersensitivity to terbinafine or to any component of the drug;
- breastfeeding period;
- children under 12 years of age (for this dosage form).
Directions for use and doses
The duration of treatment and dosage regimen are determined individually and depend on the location of the process and the severity of the disease.
Orally for adults, the drug is prescribed 250 mg 1 time/day (after meals).
For onychomycosis, the duration of treatment is about 6-12 weeks. For onychomycosis of the hands and feet (except for the big toe), or in young patients, the duration of treatment may be less than 12 weeks. For a big toe infection, a course of treatment of 3 months is usually sufficient. In rare cases, if the rate of nail growth is slow, longer treatment may be required - up to 6 months or more.
For dermatomycosis of the feet (interdigital, plantar or sock-type), the duration of treatment is from 2 to 6 weeks; for dermatomycosis of the legs - from 2 to 4 weeks; for dermatomycosis of the trunk - 4 weeks; for candidiasis of the skin and mucous membranes from 2 to 4 weeks.
For mycosis of the scalp caused by Microsporum canis, the recommended duration of treatment is more than 4 weeks.
For children weighing from 20 to 40 kg, the drug is prescribed at a dose of 125 mg (1/2 tablet) 1 time / day, with a body weight of more than 40 mg - 250 mg 1 time / day. The duration of treatment for mycoses of the scalp is about 4 weeks. In cases where the causative agent is Microsporum canis, treatment may be longer.
Elderly patients are prescribed the drug in the same doses as adults.
Thermikon tablets
TERMIKON®
belongs to antifungal agents from the group of allylamines.
Has a wide spectrum of antifungal action. Shows activity against the main spectrum of pathogens that cause fungal infections of the skin and its derivatives.
It disrupts the early stage of biosynthesis of the main component of the cell membrane of the fungus, ergosterol, and thereby has a fungicidal effect on fungi - dermatophytes, molds, yeasts and some dimorphic fungi. The effect on yeast fungi of the genus Candida and its mycelial forms can be fungicidal or fungistatic, depending on the type of fungus.
It has good penetrating abilities into the dermal layer of the skin and nail plates. Penetrates into the secretions of the sebaceous glands, accumulates in high concentrations in hair follicles, hair, skin and subcutaneous tissue.
It has a long half-life, which allows the use of TERMIKON ®
tablets only 1 time per day.
Indications for use of TERMIKON tablets®:
- onychomycosis
- mycoses of the scalp (trichophytosis, microsporia)
- fungal diseases of smooth skin – treatment of common dermatomycosis of the trunk and extremities
- candidiasis of the skin and mucous membranes caused by fungi of the genus Candida - in cases where the localization and prevalence of the process determine the advisability of oral therapy.
Directions for use and dosage:
The duration of the course of treatment and dosage regimen are established individually and depend on the localization of the process and the severity of the disease.
ADULTS:
The usual dose is 250 mg (1 tablet) 1 time per day.
Onychomycosis: duration of therapy is about 6-12 weeks. If the rate of nail growth is slow, longer treatment of up to 6 months or more may be required.
Fungal infections of the skin: the duration of treatment for interdigital, plantar or “sock” localization of infection is 2-6 weeks, for mycoses of other parts of the body: legs - 2-4 weeks, torso - 4 weeks; for mycoses caused by Candida, 2-4 weeks; for mycoses of the head caused by Microsporum canis - more than 4 weeks.
FOR CHILDREN:
Usually 125 g (1/2 tablet) is prescribed. The duration of treatment for mycoses of the scalp is about 4 weeks; for infection with Microsporum canis, it may be longer.
- With a weight from 20 to 40 kg - 125 mg 1 time per day.
- With a weight of more than 40 kg - 250 mg 1 time per day.
FOR ELDERLY PATIENTS:
the drug is prescribed in the same doses as for adults. When using the drug in patients in this age group, the possibility of concomitant liver and kidney dysfunction should be taken into account.
In case of severe impairment of liver and/or kidney function, creatinine clearance <50 ml/min or creatinine concentration in the blood more than 300 µmol/l): 125 mg 1 time per day.
Contraindications:
- hypersensitivity to any component of the drug;
- children under 3 years of age and with body weight up to 20 kg (for this dosage form)
- breastfeeding period.
Carefully:
For liver dysfunction, suppression of bone marrow hematopoiesis, cutaneous lupus erythematosus or systemic lupus erythematosus, psoriasis.
special instructions
It should be taken into account that irregular use or early termination of treatment increases the risk of relapse.
The duration of therapy may be determined by the presence of concomitant diseases and the condition of the nails at the beginning of the course of treatment.
If after 2 weeks of treatment with Thermikon there is no improvement in the condition, it is necessary to re-determine the causative agent of the disease and its sensitivity to the drug.
Systemic use for onychomycosis is justified only in the case of total damage to most nails, the presence of severe subungual hyperkeratosis, and the ineffectiveness of previous local therapy. When treating onychomycosis, a clinical response is usually observed several months after mycological cure and cessation of treatment, which is due to the rate of regrowth of a healthy nail. Removal of nail plates is not required when treating onychomycosis of the hands for 3 weeks and onychomycosis of the feet for 6 weeks.
During treatment, the activity of hepatic transaminases in the blood serum should be monitored. In rare cases, cholestasis and hepatitis develop after 3 months of treatment. If symptoms of liver dysfunction are detected (weakness, persistent nausea, lack of appetite, abdominal pain, jaundice, dark urine or colorless feces), the drug must be discontinued.
The drug should be prescribed with extreme caution to patients with psoriasis, since in very rare cases terbinafine can provoke an exacerbation of psoriasis.
When treating with the drug, general hygiene rules should be observed to prevent the possibility of re-infection through underwear and shoes. During the process (after 2 weeks) and at the end of treatment, it is necessary to carry out antifungal treatment of shoes, socks and stockings.
If allergic reactions develop, the drug should be discontinued.
Thermikon is contraindicated for use in children under 3 years of age and children weighing up to 20 kg.
Terbinafine does not affect the ability to drive vehicles or perform work that requires increased concentration and speed of psychomotor reactions.
Thermikon
Use during pregnancy and breastfeeding
Experience with the use of Thermikon® during pregnancy is limited, therefore its use during pregnancy is contraindicated.
Terbinafine is excreted in breast milk, so if it is necessary to prescribe the drug during lactation, the issue of stopping breastfeeding should be decided.
In experimental studies, the teratogenic effect of terbinafine was not revealed. To date, no malformations have been reported with the use of terbinafine.
Use for liver dysfunction
The drug should be administered orally and externally with caution in case of liver failure.
Use for renal impairment
Patients with renal failure are prescribed 125 mg 1 time per day orally.
Caution should be exercised when prescribing the drug externally in case of renal failure.
Use in children
Contraindicated: children under 3 years of age and children weighing up to 20 kg.
Use in elderly patients
Elderly patients are prescribed the drug in the same doses as adults.
special instructions
It should be taken into account that irregular use or early termination of treatment increases the risk of relapse.
The duration of therapy may be determined by the presence of concomitant diseases and the condition of the nails at the beginning of the course of treatment.
If after 2 weeks of treatment with Thermikon there is no improvement in the condition, it is necessary to re-determine the causative agent of the disease and its sensitivity to the drug.
Systemic use for onychomycosis is justified only in the case of total damage to most nails, the presence of severe subungual hyperkeratosis, and the ineffectiveness of previous local therapy. When treating onychomycosis, a clinical response is usually observed several months after mycological cure and cessation of treatment, which is due to the rate of regrowth of a healthy nail. Removal of nail plates is not required when treating onychomycosis of the hands for 3 weeks and onychomycosis of the feet for 6 weeks.
During treatment, the activity of hepatic transaminases in the blood serum should be monitored. In rare cases, cholestasis and hepatitis develop after 3 months of treatment. If symptoms of liver dysfunction are detected (weakness, persistent nausea, lack of appetite, abdominal pain, jaundice, dark urine or colorless feces), the drug must be discontinued.
The drug should be prescribed with extreme caution to patients with psoriasis, since in very rare cases terbinafine can provoke an exacerbation of psoriasis.
When treating with the drug, general hygiene rules should be observed to prevent the possibility of re-infection through underwear and shoes. During the process (after 2 weeks) and at the end of treatment, it is necessary to carry out antifungal treatment of shoes, socks and stockings.
If allergic reactions develop, the drug should be discontinued.
Use in pediatrics
Thermikon® is contraindicated for use in children under 3 years of age and children weighing up to 20 kg.
Impact on the ability to drive vehicles and operate machinery
Terbinafine does not affect the ability to drive vehicles or perform work that requires increased concentration and speed of psychomotor reactions.