Compound
Contains the active ingredient terbinafine in the form of terbinafine hydrochloride.
1 tablet of Terbizil contains 125 or 250 mg of this substance, as well as auxiliary components: hypromellose, silicon dioxide, sodium carboxymethyl starch, magnesium stearate, microcrystalline cellulose.
1 gram of Terbizil cream contains 8.8 mg of the active ingredient, as well as isopropyl myristate, sodium hydroxide, benzyl alcohol, cetyl alcohol, cetostearyl alcohol, sorbitan stearate, cetyl palmitate, polysorbate, and pure water.
Release form and composition
Dosage form of release of Terbizil:
- tablets: round, biconvex, almost white or white in color, on one side there is a mark, on the other there is an engraving “250” (14 pieces in blisters, 1 or 2 blisters in a cardboard pack);
- cream for external use 1%: almost white or white, with a slight odor of almonds (in aluminum tubes of 15 g, 1 tube in a cardboard pack).
Composition of 1 tablet:
- active substance: terbinafine – 250 mg (terbinafine hydrochloride – 281.25 mg);
- auxiliary components: sodium carboxymethyl starch (type A) - 44.85 mg, hypromellose - 11.7 mg, colloidal silicon dioxide - 3.9 mg, microcrystalline cellulose - 44.4 mg, magnesium stearate - 3.9 mg.
Composition of 100 mg cream:
- active substance: terbinafine – 1 mg (terbinafine hydrochloride – 0.88 mg);
- excipients: sodium hydroxide – 0.12 mg, benzyl alcohol – 1 mg, cetyl palmitate – 2 mg, sorbitan monostearate – 1.9 mg, cetostearyl alcohol (cetyl alcohol – 60%, stearyl alcohol – 40%) – 4 mg, cetyl alcohol – 4 mg, isopropyl myristate – 8 mg, polysorbate 60 – 6.1 mg, purified water – 71.88 mg.
Pharmacodynamics and pharmacokinetics
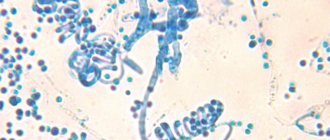
Terbizil is a broad-spectrum antimycotic and belongs to the group of allylamines. The active ingredient is terbinafine .
In low concentrations, the main substance exhibits a fungicidal effect, to which fungi (Microsporum canis, Trychophyton rubrum, Epidermophyton floccosum, etc.), mold fungi, as well as fungi of the genus dimorphic (Pityrosporum orbiculare) are susceptible.
Terbizil has both a fungicidal and fungistatic effect against yeast fungi.
The fungicidal effect is achieved by suppressing sterol synthesis in cells, which leads to inhibition of ergosterol , and this in turn causes the death of the microorganism.
Additionally, Terbizil causes inhibition of the enzyme squalene epoxidase . Squalene epoxidase is located in the cell membrane, and disruption of its work leads to the accumulation of squalene in the cell and the death of the fungus.
For humans, Terbizil is not at all dangerous, since terbinafine does not have a similar effect in the cells of the human body.
Indications for use
Mycotic infections of the skin and nail plate, diseases of the skin and hair of the head of a fungal nature ( onychomycosis , microsporia , trichophytosis , epidermophytosis , cutaneous candidiasis, rubrophytosis, mucosal candidiasis); lichen versicolor (only forms for local use are used).
Directions for use and dosage
The duration of the course is determined by the clinical picture of the disease and the severity of the condition.
Pills
Terbizil is taken orally after meals.
Frequency of application – 1 time per day.
Standard dosage regimen: 1 tablet per day, daily.
Course duration:
- onychomycosis: 6–12 weeks. In some cases, if the nails are damaged, especially with slow nail growth, the duration of therapy can be increased. For lesions of the fingernails, the course lasts about 6 weeks, for the toenails, especially the thumb - 12 weeks. The duration of use may be influenced by the age of the patient, as well as the presence of concomitant diseases and the condition of the nails before starting the drug. As a rule, clinical recovery occurs several months after negative mycological results, which is usually associated with the rate of regrowth of a healthy nail;
- mycoses of smooth skin: interdigital, plantar or sock-type localization of infection - from 2 to 6 weeks; skin candidiasis – 2–4 weeks; mycoses of the legs/torso – 2–4 weeks; candidiasis of the scalp, as well as M. canis infections – 4 weeks.
When treating mycoses of the scalp for children, Terbizil is prescribed in adult doses.
Daily doses in children:
- 12–20 kg: 62.5 mg;
- 20–40 kg: 125 mg;
- > 40 kg: 250 mg.
For patients with renal failure, Terbizil is prescribed in a reduced dose - 125 mg.
Cream
Terbizil is applied externally, after preliminary cleaning and drying of the affected areas. The drug should be applied in a thin layer. The cream is lightly rubbed into the affected and adjacent areas of the skin. It is recommended to cover physiological skin folds (interdigital spaces, groin/intergluteal areas, areas under the mammary glands) with gauze after applying the cream, especially at night.
Standard dosage regimen for patients of all ages: 1-2 times a day.
Course duration:
- dermatomycosis of the legs and torso, skin candidiasis: 7–14 days;
- dermatomycosis of the feet: 14–28 days;
- tinea versicolor: 14 days.
Contraindications
There are no absolute contraindications for the use of Terbizil cream and tablets; the only possible one is hypersensitivity to terbinafine.
Relative (Terbizil should be prescribed with caution) contraindications: pathology of the hepatic system and kidneys, accompanied by impaired organ function and failure.
You should be careful if the patient has diseases of the hematopoietic organs and endocrine diseases, vascular and oncological pathologies .
There is no experimental evidence of the safety of terbinafine in pediatric practice; before reaching the age of two, the drug is used according to strict indications.
Terbizil should not be prescribed during breastfeeding or pregnancy.
special instructions
Failure to comply with the prescribed dosage regimen (irregular use, early termination of therapy) may lead to relapse of the disease.
Pills
Taking Terbizil orally against lichen versicolor is ineffective.
For onychomycosis, systemic therapy is justified only in cases of total damage to most nails, the presence of severe subungual hyperkeratosis, and also in the absence of effect from local therapy.
Before starting treatment, it is necessary to analyze the indicators of the functional state of the liver, and during the use of Terbizil, its condition must be frequently and regularly monitored.
If liver disorders associated with therapy develop, the drug is discontinued.
In patients with severely impaired renal function (with creatinine clearance <50 ml/min), Terbizil is prescribed in a halved dose.
It must be taken into account that the clinical effect often appears only after several weeks of use of the drug.
In order to prevent re-infection through shoes/underwear, during the course of treatment you must follow the rules of hygiene and promptly carry out antifungal treatment.
Cream
Contact of Terbizil with mucous membranes should be avoided. In case of accidental contact, they should be washed with copious amounts of water (consultation with a specialist may be required).
The clinical effect of using the cream is usually observed from the first days of the course. If after 2 weeks of treatment no improvement is observed, it is recommended to clarify the diagnosis and check the sensitivity of the pathogen to the action of Terbizil.
Side effects
With a frequency of up to 10%, disorders of the digestive tract are possible: loss of appetite, nausea, diarrhea, pain in the epigastric zone.
Terbizil can cause allergic skin reactions (urticaria, rash). There have been cases where pain in the joints and muscles appeared while taking the drug.
The nervous system may react to taking the drug with dizziness. Possible headache.
Extremely rare, but still not excluded, are taste disturbances, up to its complete loss, congestion in the hepato-pancreatic-duodenal region ( cholestatic jaundice ), polymorphic exudative erythema, bullous dermatitis up to toxic epidermal necrolysis ( Lyell's syndrome ).
Signs of inhibition of the function of hematopoietic organs may be observed: agranulocytosis and thrombocytopenia , lymphopenia or neutropenia.
hyperemia may occur at the site of application .
Instructions for use of Terbizil (Method and dosage)
The duration of therapy is largely determined by the nature of the disease and its severity.
Terbizil tablets, instructions for use
In pediatrics, in the treatment of children, starting from the third year of life, a dosage of 125 mg is used, prescribed once (per day).
For adults, a dosage of 250 mg should be used; it is recommended to take this amount of Terbizil in one dose.
For onychomycosis, the duration of an effective course of treatment is from six to twelve weeks, determined by the growth rate of a healthy nail plate.
The duration of treatment may be determined by other factors: age, presence of concomitant pathology.
Terbizil is used in the treatment of dermatomycosis of the feet (plantar, interdigital or sock-type) - the treatment period is from two to six weeks; for dermatomycosis of other areas of the skin, the course of therapy lasts from two to four weeks; for head diseases of mycotic etiology - 4 weeks.
Instructions for Terbizil cream (ointment)
The cream is applied 1-2 times a day. It is necessary to clean and dry the lesions before applying Terbizil. The cream is distributed in a thin layer, covering not only the lesion, but also the adjacent areas of healthy skin.
For fungal infections accompanied by diaper rash, it is recommended to cover the cream with a bandage.
Instructions for use TERBISIL® tablets
Unlike topical treatment with Terbizil® cream, Terbizil® tablets are ineffective in treating tinea versicolor caused by Malassezia furfur. Terbizil is ineffective against vaginal candidiasis.
Terbizil® for oral administration is prescribed when it is not possible to use a dosage form for topical use (for example, localization, severity, size of the fungal lesion).
When prescribing terbinafine orally, the presence of liver disease should be taken into account. Pharmacokinetic studies in patients with liver disease have shown that the clearance of terbinafine in these patients may be reduced by 50%. Patients receiving terbinafine by mouth should be instructed to immediately report any signs or symptoms suggestive of liver dysfunction, such as persistent unexplained nausea, lack of appetite or weakness, vomiting, upper right abdominal pain, jaundice, dark urine, light feces. Patients with such symptoms should stop taking Terbizil tablets. Monitoring of liver function indicators is necessary.
Terbizil® should be used with caution in patients with psoriasis; in very rare cases, exacerbation of psoriasis is possible.
Laboratory studies have shown that terbinafine inhibits CYP2D6-mediated metabolism. Therefore, caution is required when coadministering drugs that are primarily metabolized by this enzyme (eg, tricyclic antidepressants, beta-blockers, selective serotonin reuptake inhibitors, MAO type B inhibitors and class IC antiarrhythmics). Dosage adjustments may be required.
Impact on the ability to drive vehicles and operate machinery
There is no evidence that terbinafine affects the ability to drive vehicles.
Experimental results
In long-term studies (up to 1 year) in rats and dogs, no significant toxic effects were observed in either species up to a dose of 100 mg/kg/day. At high oral doses, the liver and possibly the kidneys have been identified as target organs.
In a two-year carcinogenicity study in mice receiving terbinafine doses of 130 (male) and 156 (female) mg/kg/day, no tumors occurred. In a 2-year oral carcinogenicity study in rats, an increase in the incidence of liver neoplasms was observed in males at the maximum dose level of 69 mg/kg/day. At this dose level, systemic exposure was close to clinical. The mechanism of tumor development or its clinical significance is unknown.
Changes that may be associated with peroxisome proliferation have shown to be species dependent as they were not observed in carcinogenicity studies in mice, dogs, or monkeys.
In a high-dose study in monkeys, retinal abnormalities were observed at the highest doses (non-toxic exposure level of 50 mg/kg). These disorders were associated with the presence of terbinafine metabolites in the ocular tissues; they disappeared after discontinuation of the drug. No histological changes were noted.
A standard battery of in vitro tests and in vitro genotoxicity tests revealed no evidence of mutagenic or clastogenic potential.
Interaction
Terbizil affects the clearance of drugs whose metabolism occurs through the cytochrome P450 (tolbutamide, cycloserine , oral contraceptives), and terbinafine can both accelerate and slow down the metabolism of these drugs.
The metabolism of Terbizil itself can be accelerated by substances that enhance the activity of microsomal enzymes of liver cells ( rifampicin ).
Drugs that inhibit the activity of the cytochrome P450 enzyme slow down the elimination of terbinafine, which requires adjustment of the dose of the latter.
Drug interactions
Pills
When Terbizil is used in combination with certain drugs/substances, the following effects may develop:
- tricyclic antidepressants, selective serotonin uptake blockers, MAO-B blockers, β1-blockers: Terbizil interferes with their metabolism; the combination requires caution, especially in the case of a narrow therapeutic index;
- CYP450 inhibitors, including cimetidine: the elimination of terbinafine is slowed down (dose adjustment may be required);
- inducers of CYP450 enzymes, including rifampicin: the elimination of terbinafine is accelerated (dose adjustment may be required);
- caffeine: its clearance is significantly reduced;
- ethanol and other hepatotoxic drugs: the likelihood of developing a hepatotoxic effect increases.
Cream
The interaction of Terbizil with other drugs/substances is unknown.
Analogs
Level 4 ATX code matches:
Mikonorm
Undecin
Gentian violet
Zinkundan
Exoderil
Exiter
Batrafen
Lotseril
Lamisil Dermgel
Lamisil Uno
Lamisil
Salicylic acid
Salicylic ointment
Keto Plus
Exifin
Fungoterbin
Mycoseptin
Thermikon
Nitrofungin
Sulsena
Analogues include the following drugs: Atifan , Binafin , Lamisil , Lamikon , Lamifast , Lamifen , Mikonorm , Mikofin , Terbinafine , Terbinox , Terbinorm , Fungoterbin , Exifin .
Terbizil price, where to buy
The price of Terbizil ointment is about 350 rubles.
The price of Terbizil in tablets of 250 mg is in the range of 890-1300 rubles per pack of 14 pieces.
- Online pharmacies in RussiaRussia
- Online pharmacies in UkraineUkraine
- Online pharmacies in KazakhstanKazakhstan
ZdravCity
- Terbizil cream 1% 15g JSC Gedeon Richter
RUB 261 order
Pharmacy Dialogue
- Terbizil cream (tube 1% 15g)Gedeon-Richter
RUB 279 order
show more
Pharmacy24
- Terbizil 1% 15g cream VAT "Gedeon Richter", Ugorshchina
157 UAH. order
PaniPharmacy
- Terbizil cream Terbizil cream 1% 15g Hungary, Gedeon Richter
164 UAH order
show more