Description and composition
The drug Dorzopt is produced in the form of a clear, odorless liquid. The solution has a viscous consistency. The drug contains 2 active substances, namely:
- dorzolamide;
- Timolol
The list of auxiliary components includes:
- hyethylosis;
- citric acid monohydrate;
- sodium hydroxide;
- mannitol;
- benzalkonium chloride;
- sodium hydroxide solution;
- hydrochloric acid solution;
- purified water.
The listed components ensure increased effectiveness of the medicinal composition. The constituent elements can also be considered as preservatives, ensuring the storage of active substances for the period declared by the manufacturer.
Pharmacological group
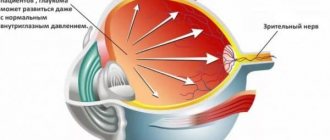
The active substances provide a blockade of m-cholinergic receptors to ensure tension in the ciliary muscle of the eye and simultaneous relaxation of the muscles that constrict the pupil. Relaxation of the ciliary muscles of the eye leads to the development of paralysis of accommodation, cycloplegia. Temporary paralysis of accommodation ensures the restoration of lost eye functions in various diseases, and makes it possible to distinguish the normal phase from true myopia at the time of diagnosis.
Dosopt easily penetrates the conjunctiva of the eye. The active component reaches its peak pharmacological activity half an hour after instillation. The pupil remains dilated for 10 hours after instillation, in some cases more. Residual effects of accommodation paresis may persist for 24 hours after instillation. The composition has antispasmodic properties and reduces the secretion of gastric glands. The drug in therapeutic doses has a stimulating effect on the nervous system. The effect of using the composition can be seen 30-60 minutes after instillation. The drug provides an increase in intraocular pressure and a decrease in the tone of the vagus nerve. Such activity leads to the development of tachycardia and a slight increase in blood pressure.
Timolol - the compound is a non-selective beta-adrenergic receptor blocker without sympathomimetic activity. When used topically, it helps to reduce the secretion of intraocular fluid and to a small extent enhances its outflow from the chambers of the eye. The component does not affect the functional state of the cardiovascular system.
Dorzolamide is a compound that selectively inhibits the functional activity of the carbonhydrase II enzyme, thereby reducing the production of intraocular fluid and reducing pressure.
What medications does Dorzopt Plus consist of?
The composition of Dorzopt Plus drops includes substances such as timolol and dorzolamide. The drug helps normalize intraocular pressure.
Dorzolamide, present in the drug, belongs to the selective inhibitors of carbonic anhydrase type II. Another component of the drug, timolol, is a beta-blocker. This substance activates the outflow of intraocular fluid. A pronounced effect from the use of Dorzopt Plus drops is observed twenty minutes after using the drug.
Indications for use
The main indication for the use of the drug is increased intraocular pressure in open-angle glaucoma and pseudoexfoliative glaucoma with insufficient effectiveness of monotherapy.
for adults
Drops are prescribed to reduce intraocular pressure in open-angle glaucoma and intraocular hypertension in cases where monotherapy has not given the necessary therapeutic effect. The drug has high criteria for effectiveness and safety, but can only be used as prescribed by a doctor. Independent use is unacceptable due to the high risk of adverse reactions. In compliance with the rules of increased caution, the medication is prescribed to elderly patients. If the rules of use are followed, the likelihood of absorption of the substance into the systemic circulation remains minimal.
for children
Children under 18 years of age are considered a stable contraindication to the use of the drug Dorzopt. The medicinal composition can provoke the appearance of permanent deformations in the structures of the eye.
for pregnant women and during lactation
The drug should not be used during pregnancy and breastfeeding. In some cases, when the expected benefit to the mother significantly exceeds the risks to the fetus, the composition can be used during pregnancy. The regimen of use is determined privately, and the woman's condition must be strictly monitored.
Contraindications
The list of contraindications to the use of the drug Dorzopt is quite extensive and includes several items:
- Severe chronic obstructive pulmonary pathology.
- Sinus bradycardia.
- Increased bronchial reactivity.
- Atrioventricular block 2-3 degrees.
- Cardiogenic shock.
- Severe insufficiency of functional activity of the kidneys with a significant slowdown in the excretion of metabolites of active substances.
- Concomitant use with oral dosage forms of drugs from the carbonic anhydrase inhibitor group.
- Age up to 18 years.
- Severe lack of functional activity of the heart.
- Severe allergic rhinitis.
- Angle-closure glaucoma.
- Pregnancy at any stage and lactation period.
- Hyperchloremic acidosis.
- Individual intolerance to the components of the drug.
- Bronchial asthma.
Using the product for patients with similar conditions and disorders is dangerous. The drug may cause severe adverse reactions. In some cases, the use of the drug may cause the patient to lose vision.
Applications and dosages
The dosage regimen is determined privately and largely depends on the indications for use. Dorzopt drops are intended for one-time instillation into the eyes, 1-2 drops. According to the instructions, if fundus examination is necessary, patients are prescribed 1 drop of the drug three times into the eye, with an interval of 10 minutes. For the treatment of infectious and inflammatory eye diseases, drugs are used in a dose of 1-2 drops of the drug into the conjunctival sac 4 times a day. The interval between uses should be equal. The duration of treatment is about 7 days. If there are no results or the patient’s condition worsens, you should contact a specialist to correct the diagnosis and treatment.
for adults
The average recommended therapeutic dose is 1 drop, which is instilled into the lower conjunctival sac 2 times a day. If eye drops are used to replace another drug, then a break of 24 hours is provided beforehand.
for children
The drug is prohibited for use in patients of this age group. The drug can provoke severe adverse reactions in patients in this group. The drug composition can cause vision loss when used in high doses.
for pregnant women and during lactation
Women during pregnancy and lactation are prohibited from using the drug. In some cases, when the risk of a woman's vision loss exceeds the expected damage to the fetus, the composition is used. In this case, the woman is recommended to use the medication for a limited course - this condition helps prevent the likelihood of adverse reactions.
Use during pregnancy and lactation
Studies on rats have shown the teratogenic effect of dorzolamide. It has not been established whether the substance penetrates into the breast milk of lactating women, however, a decrease in body weight gain was noted in the cubs of lactating female rats.
There is insufficient information on the use of timolol during pregnancy. Epidemiological studies did not reveal the effect of beta-blockers on the formation of congenital malformations of the fetus, but intrauterine growth retardation was detected. In addition, in newborns whose mothers received beta-blockers before birth, symptoms of beta-adrenergic receptor blockade were diagnosed: hypoglycemia, arterial hypotension, bradycardia, respiratory failure. Beta blockers are also known to pass into breast milk.
Taking into account the above, the use of Dorzopt Plus is contraindicated during pregnancy and lactation. If it is necessary to prescribe the drug to a nursing woman, breastfeeding should be stopped.
Side effects
During the use of the drug, adverse reactions are often observed, which are expressed in the form of local changes: signs of irritation, including a burning sensation, “sand in the eyes”, redness of the cornea, inflammation of the eyelids, transient point erosion of the corneal surface, swelling of the corneal tissue, change in the direction of eyelash growth , which can increase eye irritation, increased pigmentation of the iris, and changes in eyelashes. In patients with a predisposition to the development of allergic reactions, the following changes can be observed: attacks of bronchial asthma, which mainly develop in patients with an unfavorable medical history, shortness of breath. With prolonged use, patients run the risk of experiencing the following symptoms: headaches of varying severity, periodic dizziness.
Side effects
Dorzopt Plus eye drops, the instructions for which are included in the cardboard packaging, may cause unwanted reactions.
Due to the presence of dorzolamide in the drug, the following side effects occur:
- lacrimation, inflammation of the eyelid, peeling and irritation of the eyelid, punctate keratitis, iridocyclitis, irritation of the pharynx, transient myopia, which disappears after discontinuation of the drug;
- dry mouth, angioedema, rash;
- urticaria, bronchospasm, itching;
- nosebleeds, headaches, asthenia, paresthesia, fatigue.
As for such an active substance as timolol, it provokes the following reactions:
- conjunctivitis, blepharitis, paresthesia, keratitis, decreased sensitivity of the cornea, heart rhythm disturbances, changes in the refractive power of the eye, ptosis, diplopia;
- ringing in the ears, headache, dry eye syndrome, memory loss, asthenia, nightmares, fatigue, depression, dizziness, increasing signs of myasthenia gravis, insomnia;
- Raynaud's syndrome, visual disturbances, fainting, arrhythmia, cardiac arrest, decreased blood pressure, swelling, decreased temperature of the legs and arms;
- bronchospasm, chest pain, cough;
- alopecia, exacerbation of psoriasis, psoriasis-like rash;
- anaphylaxis, urticaria, angioedema, generalized or local rash;
- diarrhea, dry mouth, dyspepsia;
- decreased libido, systemic lupus erythematosus, Peyronie's disease.
special instructions
Patients should pay attention to the following conditions:
- The active components of the drug do not directly affect the functional state of the cerebral cortex and other structures of the central nervous system.
- After instillation, a temporary decrease in visual acuity is possible, so it is recommended to refrain from engaging in potentially hazardous activities (driving a car) for 15 minutes.
- The use of the drug for the treatment of angle-closure glaucoma, the development of which requires other therapeutic approaches, is not recommended.
- It is not recommended to touch the tip of the dropper bottle to surrounding objects, as this significantly increases the risk of infection of the tissues of the eye structures.
- Discontinuation of the drug should be carried out by gradually reducing the recommended therapeutic dose.
- The use of the drug in patients with concomitant corneal defects or after surgery on the eye can lead to decompensation of its functional state and the development of severe edema.
- Long-term use of drugs containing timolol can lead to the development of resistance.
- Patients are advised to temporarily stop wearing contact lenses, since the drug contains benzalkonium chloride, which has a negative effect on their condition. If the patient continues to wear lenses, they should be removed before each instillation of the drug; reinstallation is allowed no earlier than after 15 minutes.
- If it is necessary to perform surgical intervention, the anesthesiologist should be warned about the possible use of the drug, since adjustments to the anesthetic agents may be required.
Compliance with the above instructions ensures proper operation of the medication.
Special Recommendations
Before prescribing the eye drug Dorzopt Plus, the patient should ensure adequate monitoring of the condition of the heart and vascular system. People with pathologies in this area should be under constant supervision of specialists.
The medication in question contains the preservative benzalkonium chloride. It can settle on soft contact lenses and cause damage to the tissues of the visual organs. Therefore, people who use vision-improving products must remove them before using the drops and install them only 20 minutes after instillation of the drug.
Dorzopt Plus is prescribed with extreme caution to people with diabetes. This is due to the fact that beta blockers mask the signs of acute hypoglycemia.
Before undergoing elective surgery, it is necessary to gradually withdraw the medication two days before general anesthesia, since beta blockers can enhance the effect of muscle relaxants.
Overdose
The likelihood of overdose symptoms in patients. The use of the composition for instillation into the eyes remains minimal. The risk of adverse reactions increases with accidental oral administration of the medication. The patient may experience the following changes:
- dry skin and mucous membranes;
- tachycardia;
- mydriasis;
- convulsions;
- depression of consciousness, coma;
- accommodation paresis;
- stomach ache;
- headache.
Treatment begins with gastric lavage and taking sorbents. Subsequently, symptomatic treatment is carried out. It is important to take the patient to the hospital if symptoms of acute intoxication are intense.
Analogs
The composition of Dorzopt Plus does not have full-fledged analogues that provide a complete replacement. As a rule, to ensure such an effect, doctors recommend the use of several local remedies. The drug replacement regimen is determined privately and adjusted by the doctor as the patient recovers.
Cyclomed
The drug Cyclomed is used for external use in ophthalmology as eye drops. The drug is produced in the form of a weakly concentrated solution. The drug causes separation of the ciliary muscle of the eye and leads to the development of paralysis of accommodation. Temporary paralysis ensures the restoration of the natural function of the eye. The active substance quickly and easily penetrates the membrane. The active substance does not penetrate the systemic bloodstream, which makes the composition safer.
Midrimax
Midrimax is a drug with a combined effect. The drug contains several active components. The main purpose of using Sotava is pupil dilation, which may be necessary for various ophthalmological reasons, both diagnostic and therapeutic. Can be used from the age of 18. The pharmacological effect occurs quickly and lasts for several hours.
Azarga
The drug Azagra is produced by pharmaceutical companies in the form of eye drops. The composition provides treatment for glaucoma in adult patients. Drops are contraindicated for children, adolescents under 18 years of age, pregnant and breastfeeding women.
Kosopt
The drug Kosopt is produced in the form of eye drops and is considered as a complete substitute for the drug Dorzopt. The drug provides rapid elimination of side symptoms of glaucoma and increases the effectiveness of the main therapy. The drug is contraindicated in children and adolescents under 18 years of age, as well as pregnant and lactating women.
Pharmacodynamics and pharmacokinetics
The drug is a local carbonic anhydrase . The mechanism of action is based on inhibition of the secretion of intraocular fluid and slowing down the formation of bicarbonate with a subsequent weakening of the transfer of sodium and water.
The drug does not cause spasm of accommodation, as well as miosis or hemeralopia.
Pharmacokinetic information
- Absorption occurs through the cornea, and also, to a lesser extent, through the sclera and limbus. Systemic absorption rates are low. Once in the blood stream, it quickly penetrates into red blood cells with a significant content of carbonic anhydrase II.
- Binding to plasma proteins occurs by 33%. Converts to an N-desethylated metabolite , which is less active towards carbonic anhydrase II , but is capable of blocking carbonic anhydrase I. Long-term use leads to accumulation in red blood cells.
- Excreted through the kidneys. Withdrawal of the drug has two phases of elimination: fast and subsequent slow, due to the gradual release of the substance from red blood cells.
- Half-life: approximately 4 months.