Pharmacological properties of the drug Clexane
Low molecular weight heparin (average molecular weight about 4500 Da), in which the antithrombotic and anticoagulant activities of standard heparin are separated. Unlike standard unfractionated heparin, it is characterized by high anti-Xa activity (100 IU/ml) and weak anti-IIa or antithrombin activity (28 IU/ml). When used in recommended doses, enoxaparin sodium does not increase bleeding time. In prophylactic doses, enoxaparin sodium does not lead to significant changes in activated partial thromboplastin time (aPTT), and also does not affect platelet aggregation and fibrinogen binding to platelets. The pharmacokinetic parameters of the drug are assessed by changes in anti-Xa and anti-IIa activity in blood plasma over time in the recommended dose ranges. When administered subcutaneously, Clexane is quickly and almost completely absorbed. Absorption is directly proportional to the administered dose and is linear. The bioavailability of enoxaparin sodium when administered subcutaneously approaches 100%. Maximum anti-Xa activity in blood plasma is observed between the 3rd and 5th hour after subcutaneous administration and averages 0.18±0.04 IU/ml after administration of 2000 anti-Xa IU and 0. 43±0.11 IU/ml after administration of 4000 anti-Xa IU, and 1.01±0.14 IU/ml after administration of 10,000 anti-Xa IU. Maximum anti-IIa activity is observed on average 4 hours after subcutaneous administration at a dose of 4000 anti-Xa IU, while at the same time, when administered at a dose of 2000 anti-Xa IU, this activity cannot be determined by the traditional amidolytic method. The volume of distribution of enoxaparin sodium for anti-Xa activity almost corresponds to the volume of circulating blood. Metabolism of enoxaparin sodium occurs in the liver by desulfation and/or depolymerization to form low molecular weight heparin species with significantly lower biological potential. The half-life of anti-Xa activity corresponds to approximately 4 hours with a single dose and 7 hours with repeated administration. Anti-Xa activity is detectable up to approximately 24 hours after subcutaneous administration of 4000 anti-Xa IU enoxaparin sodium. Renal clearance of active metabolites is 10%, total renal excretion is 40% of the drug dose. Elimination of enoxaparin is longer in the elderly (half-life is 6–7 hours). In patients with renal failure (creatinine clearance ≤30 ml/min), AUC increases significantly (by 65%) with repeated administration of 4000 anti-Xa IU once daily. Pharmacokinetic parameters in patients with renal failure on hemodialysis do not change.
Reviews
- Oksana, 32 years old. She underwent treatment with Clexane on an IVF protocol. The drug is very convenient to use; thanks to the thin short needle, administering the medicine is quick and almost painless. I gave the injections on my own, but the first time I was taught how to do this by a nurse in a hospital. I did not have any complications from using Clexane, except that the bruises began to appear much easier, but I did not have any spontaneous hemorrhages throughout the entire course of therapy.
- Olga, 49 years old. She received Clexane therapy before an upcoming operation to remove varicose veins in her right leg. The course of thrombosis prevention was quite easy and without complications. The medicine is injected into the subcutaneous fat of the abdomen. The injections were well tolerated, there were no bruises at the injection site, the administration of the drug was somewhat painful, but quite tolerable. It is very pleasing that the drug is sold immediately in syringes and everything happens as accurately and sterilely as possible. There is definitely an effect from this medicine; there were no thrombotic complications after the operation.
Indications for use of the drug Clexane
Prevention of venous thrombosis and embolism during orthopedic or general surgical operations, as well as in therapeutic patients on bed rest due to acute illnesses (heart failure of functional class III–IV according to the NYHA classification, respiratory failure, severe acute infectious process, rheumatic diseases) ; prevention of thrombus formation in the extracorporeal circuit during hemodialysis; treatment of deep vein thrombosis, including those accompanied by pulmonary embolism; treatment of unstable angina and acute myocardial infarction without a pathological Q (in combination with acetylsalicylic acid).
Use of the drug Clexane
The drug is used only in adults. For prophylactic and therapeutic use, enoxaparin is administered deeply subcutaneously. The drug is administered intravenously to achieve anticoagulation during hemodialysis. Enoxaparin cannot be administered intramuscularly! Clexane is injected into the antero- or posterolateral area of the abdominal wall. The syringe needle is inserted over its entire length in a perpendicular direction to the surface of the skin fold, which is formed using the thumb and index finger and held throughout the entire injection. The patient should be in a supine position. 1 mg of enoxaparin sodium (0.01 ml solution) corresponds to approximately 100 anti-Xa IU activity. For the prevention of venous thrombosis and thromboembolism during operations with a moderate risk of thrombus formation (abdominal surgery) and in patients with a moderate risk of thromboembolism, the drug is recommended to be administered subcutaneously at a dose of 2000 anti-Xa IU once a day. For operations with a high risk of thromboembolism (surgeries on the hip or knee joint and oncological interventions), the drug is administered subcutaneously at a dose of 4000 anti-Xa IU once a day. In general surgical practice, the first dose of the drug is administered 2 hours before surgery. In orthopedic practice, the first dose of the drug is administered 12 hours before surgery. The duration of prophylactic use is on average 7–10 days. In orthopedics, it is used in a dose of 4000 anti-Xa once a day for up to 4 weeks. In immobilized medical patients at high risk of thromboembolism, the recommended dose is 4000 anti-Xa IU once daily for at least 6 days, but not more than 14 days. To prevent thrombus formation in the extracorporeal circuit during hemodialysis, the drug is used at a dose of 100 anti-Xa IU/kg of patient body weight. Enoxaparin is injected into the arterial line of the hemodialysis circuit before the start of the session. As a rule, the indicated dose is sufficient for dialysis for 4 hours; if fibrin rings appear, an additional dose of 50–100 anti-Xa IU/kg may be administered. For patients with a high risk of bleeding, the dose of the drug should be reduced to 50 anti-Xa IU/kg with dual vascular access and to anti-Xa IU/kg with single access. When fibrin rings appear, an additional dose of 50 to 100 anti-Xa IU/kg is administered. In the treatment of deep vein thrombosis, which is accompanied or not accompanied by pulmonary embolism, enoxaparin sodium is administered subcutaneously at a dose of 150 anti-Xa IU/kg once a day or at a dose of 100 anti-Xa IU/kg 2 times a day every 12 h. The duration of treatment should not exceed 10 days. If necessary, oral anticoagulants are simultaneously prescribed. Treatment is continued until the international normalization ratio (INR) reaches 2–3. Q wave in the acute phase, enoxaparin sodium is administered subcutaneously at a dose of 100 anti-Xa IU/kg every 12 hours (in combination with acetylsalicylic acid at a dose of 100–325 mg orally once a day) . The duration of treatment is 2–8 days until the patient’s condition is clinically stabilized. There is no need for dose adjustment in elderly patients with normal renal function. The use of enoxaparin sodium in children is not recommended. In patients with renal failure (creatinine clearance ≤30 ml/min), dose adjustment of the drug is required, since its effect in this category of patients is significantly enhanced. For prophylactic purposes, such patients are prescribed the drug in a dose of 20 mg (2000 anti-Xa IU) once a day, for therapeutic purposes - 1 mg/kg (100 anti-Xa IU/kg) once a day. Administration of the drug to patients with liver failure requires medical supervision.
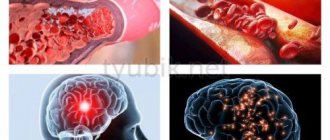
Why is Clexane prescribed for coronavirus?
In 25-30% of patients with moderate to severe pneumonia, the risk of blood clots increases. Small clots block the mouths of small and large vessels, disrupt the blood circulation of the respiratory system, brain and spinal cord, and heart. Muscle tissue and nerve fibers do not receive oxygen and nutrients, and they die.
Side effects of the drug Clexane
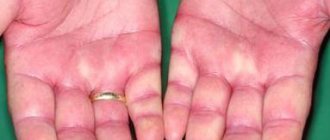
Hemorrhagic complications are possible (including isolated cases of massive bleeding, in particular retroperitoneal and intracranial; some of these cases were fatal); local or generalized allergic reactions; thrombocytopenia (mild, transient, asymptomatic thrombocytopenia in the first days of therapy; immunoallergic thrombocytopenia with thrombosis, which in some cases was complicated by organ infarction or limb ischemia); with prolonged treatment (more than 5 weeks) - early development of osteoporosis; increased activity of transaminases in blood serum; the development of neuraxial hematomas when using enoxaparin during epidural or spinal anesthesia in some cases can lead to neurological disorders of varying severity, including the formation of prolonged or permanent paralysis; reactions at the injection site (from mild irritation to pain, bruising and hematomas at the injection site, in exceptional cases - skin necrosis); skin bullous rashes or systemic allergic reactions, including anaphylactoid. If such side effects occur, treatment with the drug must be stopped. Isolated cases of hypersensitivity with cutaneous vasculitis have been reported; asymptomatic and reversible increase in platelet count and increase in liver enzyme activity.
Contraindications
The medicine should not be used in the following cases:
- with individual intolerance;
- dissecting aortic aneurysm;
- hemorrhagic stroke;
- spontaneous uncontrolled bleeding;
- reduced number of platelets in the blood serum.
Contraindications to the use of Clexane apply to pregnant women who have an artificial valve in the heart. The medication is not prescribed if the medical history contains information about impaired hemostasis, severe vasculitis, gastrointestinal diseases, ischemic stroke, arterial hypertension, and diabetes mellitus.
Special instructions for the use of the drug Clexane
Low molecular weight heparins are not interchangeable drugs, since they differ in molecular weight, specific activity against factor Xa, and dosage regimen. Clexane, like other anticoagulants, should be used with caution in conditions that are accompanied by an increased risk of bleeding, namely: with hemostasis disorders, a history of peptic ulcers, recent hemorrhagic stroke, uncontrolled severe hypertension (arterial hypertension), diabetic retinopathy, neurosurgical or ophthalmic surgical interventions, simultaneous use of drugs that affect hemostasis. During prophylactic treatment of patients over 65 years of age, no increased bleeding was observed, however, when using the drug in therapeutic doses, there may be a risk of developing hemorrhagic complications. Since there are not enough controlled clinical studies in pregnant women, enoxaparin sodium should be prescribed during pregnancy only if there is a vital indication. It is not recommended to use Clexane for the treatment of pregnant women with prosthetic heart valves. It is recommended to stop breastfeeding during treatment with the drug. Not used in pediatric practice. In patients with low body weight (less than 45 kg in women and 57 kg in men), the risk of developing hemorrhagic complications increases, which requires monitoring the patient. In order to reduce the risk of bleeding after percutaneous coronary angioplasty, the catheter providing vascular access should be removed no earlier than 6–8 hours after subcutaneous administration of enoxaparin. The next dose of enoxaparin can be administered only 6–8 hours after removal of the catheter. When performing spinal or epidural anesthesia with the use of enoxaparin sodium at a dose of 4000 anti-Xa IU/kg once a day, cases of the development of neuraxial hematomas and associated neurological disorders were rarely observed. The risk of developing such complications increases with the use of enoxaparin sodium in high doses, the use of permanent postoperative epidural catheters, or the simultaneous use of drugs that affect hemostasis, in particular NSAIDs, during repeated punctures. When combined with spinal or epidural anesthesia and enoxaparin, it is best to install and remove the catheter before administering enoxaparin. When performing spinal or epidural anesthesia, it is best to insert and remove the catheter when the anticoagulant effect of enoxaparin sodium is low: 10–12 hours after administration of a dose of 4000 anti-Xa IU/kg or less, or 24 hours after administration of the drug in high doses (100 anti-Xa IU/kg 2 times a day or 150 anti-Xa IU/kg once a day). The next administration of enoxaparin sodium should be carried out no earlier than 2 hours after removal of the catheter. Strict medical monitoring of the patient's neurological status is necessary. If signs of a spinal hematoma appear, appropriate treatment should be immediately prescribed (if necessary, spinal cord decompression). Medical supervision is required when prescribing the drug to patients with a history of heparin-induced thrombocytopenia with or without thrombosis. It is recommended to determine the platelet count before and throughout the course of treatment. If the platelet count decreases by 30–50% of the initial value, the drug should be discontinued immediately. Enoxaparin sodium in doses used for the prevention of venous thromboembolism does not significantly affect bleeding time and other parameters of blood coagulation, including platelet aggregation or fibrinogen binding to platelets. When using the drug in higher doses, the aPTT and activated clot formation time may increase. However, the increase in these indicators does not directly depend on the increase in the antithrombotic activity of enoxaparin and does not require constant monitoring.
Analogs
The active ingredient in Clexane injections prescribed during pregnancy is sodium enoxyparine, which belongs to the pharmacological group of anticoagulant substances.
Structural analogues of this drug are the following drugs:
- Hemapaxan;
- Novoparin;
- Enoxaparin sodium;
- Enoxarin;
- Anfiber.
There are drugs that have a different active ingredient, but also have an antithrombic effect:
- Fraxiparine;
- Fragmin;
- Angioflux;
- Wessel Due F.
It is important to remember that you cannot independently select a replacement for the drug recommended by your doctor, since low molecular weight heparins such as Fraxiparine or Clexane are not completely interchangeable, so during pregnancy you should not risk your health; you should listen to the doctor’s prescriptions.
These medications have different compositions, molecular weights, and have different effects on the body of a pregnant woman. Therefore, when there is a need to replace Clexane with another medicine, you should talk to your doctor about this.
To treat and prevent blood clots, doctors prescribe Clexane injections into the abdomen for women expecting to give birth. However, in the early stages of pregnancy, this drug is not recommended for use due to the fact that there is no reliable data on the absence of a negative effect on the fetus during the development of all future systems and organs. Therapy with Clexane is carried out under the strict supervision of a physician, since hemorrhagic complications may develop if the dosage of the drug is exceeded.
Author: Violeta Kudryavtseva, doctor, especially for Mama66.ru
Drug interactions Clexane
Due to the increased risk of bleeding, Clexane should not be used simultaneously with acetylsalicylic acid and other NSAIDs in high doses, ticlopidine, clopidogrel, dextran 40, corticosteroids, thrombolytics, anticoagulants, and other antithrombotic drugs, including glycoprotein IIb/IIIa antagonists. If it is necessary to use such combinations, careful clinical and laboratory monitoring should be carried out, however, today there is experience in the safe combined use of enoxaparin sodium with the above drugs.
Does it hurt to prick
Injections of the drug in ampoules, which are given into the anterior abdominal wall, are painful. Before giving birth, the doctor informs the patient about the features of the medication.
If during an injection the needle enters a blood vessel, pain occurs. After injection of the medication, bruises sometimes appear that do not go away for more than 7 days.
It may be painful for the patient if she gives injections on her own and does not know the injection technique well.
Clexane overdose, symptoms and treatment
As a specific antidote, slow intravenous administration of protamine sulfate (hydrochloride) is indicated at the rate of 1 mg of protamine per 1 mg of Clexane (if enoxaparin sodium was administered over the previous 8 hours). However, even with the introduction of protamine sulfate in a high dose, the effect of enoxaparin sodium is not completely neutralized (maximum - up to 60%). Since neutralization may be temporary (due to the absorption characteristics of low molecular weight heparins), the dose of protamine must be divided into several injections (from 2 to 4) over 24 hours.
Composition and release form
Clexane is produced in the form of injection solutions: a completely clear to pale yellow liquid in glass syringes. One cardboard package contains from 1 to 5 blisters of 2 syringes each. The official international name of Clexane is Enoxaparin, the Latin name is clexane.
The solution contains water for injection as an auxiliary component. The active ingredient is low molecular weight sodium enoxaparin. The dosage of 1 syringe is measured in international units of anti-CA ME and is:
| Syringe volume | Dose of anti-CA ME |
| 0.2 ml | 2000 |
| 0.4 ml | 4000 |
| 0.6 ml | 6000 |
| 0.8 ml | 8000 |
| 1 ml | 10 000 |