Compound
The active substance of the drug is represented by a complex of polyadenylic and polyuridylic acids 100 units :
- 0.107 mg - potassium polyribouridylate ;
- 0.1 mg - potassium polyriboadenylate .
The following are present as auxiliary components:
- 8.5 mg - sodium chloride ;
- 0.408 mg - potassium dihydrogen phosphate ;
- 2 mg - sodium hydrogen phosphate .
Description and composition
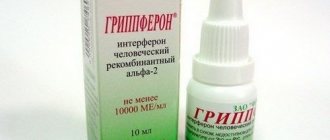
The pharmacological effect is due to a complex of amino acids that are inducers of the synthesis of endogenous interferon. One bottle contains 100 units of a mixture of polyadenylic and polyuridylic acids, as well as excipients.
Ribonucleotides were obtained by biosynthesis, while they retain their pharmacological properties and exhibit antiviral and immunomodulatory activity. The therapeutic effect is achieved due to the ability of the drug to stimulate the formation of immune defense factors, endogenous interferons, killer cells and other substances that recognize and eliminate foreign antigens.
After instillation, the medicinal solution quickly penetrates the eye tissue and begins to act directly at the site of the lesion. Its concentration is determined in tear fluid, as well as in blood serum. The production of endogenous interferon in response to the administration of Poludan begins within 3 hours. Thanks to daily instillation, immune protection in the body is maintained at a high level throughout the entire course of treatment, but quickly subsides after its completion.
Pharmacodynamics and pharmacokinetics
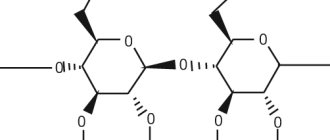
Poludan is a biosynthetic polyribonucleotide complex of polyribouridyl and polyriboadenylic . Induces the production of interferon contained in the body and other cytokines .
The mechanism of action of the complex is based on stimulation of alpha interferon to a greater extent, and beta interferon and gamma interferon to a lesser extent, as well as inducing the synthesis of interferon in blood leukocytes, organs and tissues . High levels of interferon are maintained with daily administration throughout the course of therapy.
Poludan exhibits direct antiviral activity against different strains of influenza virus and other acute respiratory viral infections . When a virus infects body cells, it triggers a cytokine response .
Indications for use
for adults
The drug is prescribed for the treatment of viral diseases in ophthalmology. Among the indications for use in the official instructions are the following:
- keratoconjunctivitis caused by adenoviral and herpes infections;
- keratitis;
- iridocyclitis;
- optic neuritis;
- deep keratitis;
- uveitis
The drug is not prescribed for bacterial infections. Its immunostimulating effect will have a positive result in the treatment only for inflammatory or viral diseases. Self-medication with Poludan is unacceptable, since it should only be prescribed by a qualified specialist after an accurate diagnosis has been determined.
for children
The drug has a high safety profile and contains a natural complex of polyribonucleotides, so it can be used in pediatrics. However, the indications for use remain the same - inflammatory eye diseases, as well as viral lesions.
for pregnant women and during lactation
The drug has a high safety profile, so it is often prescribed to pregnant and lactating women. However, there is not enough data on the use of Poldan in this category of patients.
Instructions for Poludan (Method and dosage)
The main pharmaceutical form of the drug is Poludan eye drops, instructions for use and preparation of which are presented below.
- The drug Poludan in the form of eye drops is used in adult patients for the treatment of herpetic and adenoviral conjunctivitis, keratitis and superficial keratoconjunctivitis . The prepared solution is instilled into the inner corner of the diseased eye (conjunctival sac). The recommended daily dose for adults is 6-8 instillations (for children 3-4) of 1-2 drops. As the drug acts and inflammation decreases, the number of instillations per day is gradually reduced to 3-4 times in adults and 2-3 times in children. Preparation of the solution: the contents of one bottle of the drug (200 mcg) are dissolved in 2 ml of distilled water.
- Nasal drops are prescribed to adult patients for the treatment of influenza and other acute respiratory viral infections . Instill a pre-prepared solution 5 times a day, 2 drops into each nostril. Treatment begins no later than 2 days from the moment symptoms of the disease are detected and continues for 5 days. Preparation of the solution: the contents of one bottle (100 units) are dissolved with distilled water to the mark located on the label.
- The injection solution is used for the treatment of uveitis, stromal keratitis, choreoretinitis , as well as optic neuritis of a viral nature. Injections are carried out once a day or every other day under the conjunctiva , at a dose of 100 mcg (0.5 ml of the prepared solution). A course of therapy usually requires 15 to 20 injections. When treating viral lesions of the organs of vision in children, injections are prescribed at half the dose with 8-10 injections per course of treatment. Preparation of solution: the contents of one bottle of Poludan are dissolved in 1 ml of water for injection or 1 ml of 0.5% novocaine .
Poludan
pharmachologic effect
Poludan is a biosynthetic polyribonucleotide complex of polyadenylic and polyuridylic acids (in equimolar ratios).
Inducer of endogenous interferon synthesis. Stimulates the formation mainly of alpha interferon, and to a lesser extent - beta and gamma interferons.
It has pronounced antiviral and immunomodulatory activity.
Studies have shown that instillation and subconjunctival administration of Poludan promotes the production of endogenous interferon in the blood serum and in the tear fluid of patients with ophthalmoherpes. Interferon is detected in blood serum and in tear fluid 3 hours after administration of Poludan . A high level of interferon (110 U/ml in the blood and 75 U/ml in the tear) is maintained by daily injections of Poludan throughout the course. On the 2nd day after stopping the administration of the drug, interferon is practically not detected in biological fluids (its titer does not exceed 10 U/ml).
In addition, the administration of Poludan led to a significant increase in the activity of natural killer cells, which was initially reduced in patients with ophthalmoherpes.
Studies of the influence of Poludan on immunological parameters in an in vitro system have shown that the drug stimulates not only natural cytotoxicity, but also the functioning of other immunocompetent cells, in the regulation of whose activity interferon plays a significant role.
Pharmacokinetics
Data on the pharmacokinetics of the drug Poludan are not provided.
Indications
- viral eye diseases (including adenoviral and herpetic conjunctivitis, keratoconjunctivitis, keratitis and keratoiridocyclitis, keratouveitis, stromal keratitis, iridocyclitis, chorioretinitis);
- optic neuritis (viral etiology).
Instructions for use / dosage
The drug is used in adults in the form of eye drops and injections under the conjunctiva.
In the treatment of adenoviral and herpetic conjunctivitis, superficial keratoconjunctivitis and keratitis
Poldan's solution is instilled into the conjunctival sac of the diseased eye, 1-2 drops 6-8 times a day.
As the inflammatory process subsides, the number of instillations is reduced to 3-4 times/day. In the treatment of adenoviral and herpetic conjunctivitis, stromal keratitis and keratoiridocyclitis (keratouveitis), iridocyclitis, chorioretinitis, optic neuritis
the drug is used in the form of subconjunctival injections. Inject 0.5 ml (100 mcg) under the conjunctiva of the eye daily or every other day. The course of treatment is 15-20 injections.
Rules for preparing solutions
Poludan solution , intended for instillation into the eye, is prepared by dissolving the contents of the bottle (200 mcg) in 2 ml of distilled water.
To prepare a solution for subconjunctival injection, the contents of the vial (200 mcg) are dissolved in 1 ml of sterile water for injection.
Side effect
When using the drug in the form of eye drops:
rarely - allergic reactions in the form of itching, sensation of a foreign body in the eye, increased injection of the sclera, the appearance of individual follicles in the lower transitional fold.
With subconjunctival administration:
sometimes - swelling of the lower eyelid and increased conjunctival injection.
When injected into the anterior chamber of the eye:
rarely - a short-term increase in intraocular pressure, the appearance of hemorrhages in the anterior chamber, increased tyndulation of the moisture of the anterior chamber.
Contraindications
- hypersensitivity to the components of the drug.
Use during pregnancy and breastfeeding
Data on the safety of the drug during pregnancy and lactation are not provided.
special instructions
Poludan should not be administered into the anterior chamber of the eye in case of keratoiridocyclitis with ulceration of the anterior surface of the cornea, conjunctivitis, the presence of pathogenic microflora in culture from the conjunctiva, infections of the teeth and paranasal sinuses.
Side effects disappear on their own after discontinuation of the drug within 1-3 days.
In therapeutic doses, Poludan is not pyrogenic.
The drug is compatible with antibiotics and traditional treatments for viral diseases.
Overdose
Currently, no cases of overdose of the drug Poludan have been reported.
Drug interactions
With the simultaneous use of Poludan with enzyme preparations, due to the destructive effect of enzymes on endogenous interferon, the clinical effectiveness of Poludan decreases.
Conditions for dispensing from pharmacies
The drug in the form of a lyophilized powder for the preparation of a subconjunctival solution is available with a prescription.
The drug in the form of a lyophilized powder for the preparation of eye drops is approved for use as an over-the-counter product.
Storage conditions and periods
The drug should be stored in a dry place, protected from light, at a temperature not exceeding 4°C. Shelf life – 4 years.
The prepared solution can be stored in the refrigerator for no more than 1 week.
Analogs
The following ophthalmic agents have a similar therapeutic effect:
- Okoferon. A drug based on human interferon. It is available in powder form, from which, after dilution, eye drops are prepared. Shows immunomodulatory and antiviral effects. Not used to treat children, pregnant and lactating women. Belongs to the over-the-counter group of drugs.
- Oftalmoferon. A combination drug that, in addition to the immunomodulator interferon, also contains diphenhydramine. Shows a wide spectrum of activity, including anti-inflammatory and local anesthetic effects. It is used by instillation into the eye only for adult patients.
- Aktipol. Antiviral agent based on para-aminobenzoic acid. The drug promotes the production of interferon and activation of specific protection. Prescribed mainly to adult patients for instillation into the conjunctival sac.