Visomitin eye drops represent the pharmacological group of keratoprotectors and antioxidants intended for use in ophthalmic practice. They are used for the pathogenetic treatment of dry eye syndrome in adults. The use of the medicine in children and adolescents under 18 years of age is excluded. It is not recommended to prescribe the drug during pregnancy, as well as during lactation in women.
Pharmacological group
The drug represents the pharmacological group of keratoprotectors with antioxidant action. The main active component of the drug plastoquinonyldecyltriphenylphosphone is a chemical derivative of plastoquinone. In low concentrations, it has a pronounced antioxidant effect, which manifests itself in the binding of active radicals (fragments of organic molecules that contain unpaired electrons and have high chemical activity). The drug also has several of the following therapeutic effects:
- Stimulation of the process of tear fluid production in the corresponding glands.
- Increasing the intensity of the corneal epithelization process.
- Increasing the stability of the tear film that covers the surface of the cornea of the eye.
After instillation of eye drops, the active component has a local therapeutic effect; it is not absorbed into the systemic bloodstream.
Indications for use
The medicine is used to moisturize the cornea of the eye.
for adults
The drug is prescribed for the complex treatment of dry eye syndrome, as well as the initial stage of cataracts, when there are no irreversible changes in the cornea and lens of the eye.
for children
The drug is contraindicated for use in pediatric practice.
for pregnant women and during lactation
The possibility of use is determined by the attending physician according to strict medical indications, since there is currently no reliable data on the safety of the drug for the body of a developing fetus or infant.
Applications and dosages
Eye drops are instilled into the conjunctival sac of the eye. First, you should tilt your head back, pull down the lower eyelid with the index finger of one hand, and instill the drops with the other. In this case, it is important to try not to touch the conjunctival mucosa with the tip of the dropper bottle.
for adults
The average recommended dose for the treatment of dry eye syndrome is 1-2 drops, which are instilled into the conjunctival sac 3 times a day. The duration of the course of therapy is set individually depending on the severity and nature of the pathological process that led to excessive dryness of the cornea.
For the treatment of the initial stage of cataracts, the drug is prescribed in a dose of 1-2 drops 3 times a day for a period of about six months. During treatment, effectiveness must be monitored by an ophthalmologist, who, if necessary, will change subsequent treatment tactics.
for children
The drug is contraindicated for use in pediatric practice.
for pregnant women and during lactation
The dose, frequency of use of eye drops, as well as the duration of the course of treatment are determined by the attending physician individually.
Instructions for use
MINISTRY OF HEALTH OF THE RUSSIAN FEDERATION
INSTRUCTIONS for medical use of the drug
___Visomitin®__ name of the medicinal product
Registration number : LP-001355
Trade name : Visomitin®
International nonproprietary or generic name : Plastoquinonyldecyltriphenylphosphonium bromide
Dosage form : Eye drops
Composition per 1 ml : Active ingredient: Plastoquinonyldecyltriphenylphosphonium bromide (PDTP) 0.155 mcg Excipients: Benzalkonium chloride 0.1 mg, hypromellose 2 mg, sodium chloride 9 mg, sodium dihydrogen phosphate dihydrate 0.81 mg, sodium hydrogen phosphate dodecahydrate 1.16 mg, sodium hydroxide 1 M solution to pH 6.0 – 8.0, purified water to 1 ml.
Description Clear or slightly opalescent, colorless or slightly colored liquid.
Pharmacotherapeutic group Keratoprotective agent. Antioxidant agent.
ATX code : S01ХA
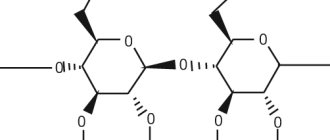
Pharmacological properties Pharmacodynamics Plastoquinonyldecyltriphenylphosphonium bromide (PDTP) is a derivative of plastoquinone, which is linked to a triphenylphosphine residue through a linker chain (C10). When used in low (nanomolar) concentrations, PDTP exhibits high antioxidant activity. It has a stimulating effect on the process of epithelization of the cornea, helps to increase the stability of the tear film.
One of the reasons for the development of age-related cataracts is the damaging effect of ultraviolet radiation, which initiates photo-oxidation processes leading to denaturation of the main structural components of the lens - crystallins. The first protection of eye tissue from ultraviolet radiation is tear fluid, which absorbs ultraviolet light in the range of 240-320 nm and neutralizes it due to the components of the antioxidant activity of tear fluid. According to preclinical studies, the anti-cataract effect of the drug Visomitin® is associated with an increase in the level of expression of the main lens proteins α-crystallins. According to a clinical study, patients with age-related cataracts who used Visomitin® showed an increase in the antioxidant activity of tears.
Pharmacokinetics Preclinical studies on animals have shown that when using Visomitin® eye drops in the form of instillations, PDTP is not detected in the blood. Following oral administration of PDTP to healthy volunteers, the plasma half-life is approximately 45 minutes.
Indications for use Including, as part of complex therapy: Symptoms of dry eye syndrome/corneal-conjunctival xerosis (poor tolerance to wind, conditioned air, smoke, foreign body sensation, pain, burning, dry eyes, floating visual acuity, lacrimation, redness of the eyes in the area not covered by the eyelids). Symptoms of the initial stage of age-related cataracts (gradual blurring and decreased visual acuity).
Contraindications Hypersensitivity to the components of the drug. Children under 18 years of age (no clinical trial data available).
Use during pregnancy and breastfeeding There is no data on the safety of using Visomitin® during pregnancy. Studies of generative function and teratogenic effect in rats did not reveal signs of impaired fertility or defects in fetal development caused by PDTF.
It is unknown whether PDTP is excreted in breast milk. Appropriate studies have not been conducted in lactating women. The use of Visomitin® during pregnancy is possible if the expected benefit to the mother outweighs the potential risk to the fetus. If you are pregnant, think you might be pregnant, or are planning a pregnancy, you should consult your doctor.
Method of administration and dosage Symptoms of “dry eye” syndrome: 1-2 drops of the drug into the conjunctival sac 3 times a day. According to clinical studies, the therapeutic effect is achieved in the first two to four weeks of use. The therapeutic effect is persistent when used for 6 weeks. If there is no effect during the first two weeks of treatment, examination by a specialist is necessary. The initial stage of age-related cataracts: 1-2 drops of the drug into the conjunctival sac 3 times a day. The duration of treatment is 6 months. The diagnosis of “initial stage of age-related cataracts” must be made by a specialist. If there is no improvement after treatment, or symptoms worsen, or new symptoms appear, you should consult your doctor. Use the drug only according to the indications, the method of use and in the doses indicated in the instructions for use.
Side effects Classification of adverse reactions by organs and systems, indicating the frequency of their occurrence: very often (≥10), often (≥1/100, <1/10), infrequently (≥1/1000, <1/100), rarely ( ≥1/10000, <1/1000), very rare (<1/10000), including isolated reports, frequency unknown (frequency cannot be estimated based on available data). On the part of the organ of vision Often: short-term pain and burning after instillation. Uncommon: temporary blurred vision. Other Often: allergic reactions (itching, redness and swelling of the eyelids and conjunctiva). If you experience the side effects listed in the instructions, or they get worse, or you notice any other side effects not listed in the instructions, tell your doctor.
Overdose When using the drug Visomitin® in accordance with the instructions for use, an overdose is unlikely. Excessive use may increase side effects. Treatment: symptomatic therapy.
Interaction with other medicinal products There have been no previously reported adverse interactions between Visomitin® and other medicinal products. If necessary, it can be used simultaneously with other eye drops; the interval between instillations should be at least 15 minutes.
Special instructions Contact lenses Visomitin® eye drops contain benzalkonium chloride as a preservative and are not recommended for use when wearing contact lenses. Before instilling eye drops, contact lenses should be removed and put back on no earlier than 15 minutes after using the drug. The bottle must be closed after each use. Do not touch the eye with the tip of the pipette while instilling. The active ingredient of Visomitin® is very sensitive to light: between uses, do not store the bottle in a lighted place! Patients using Visomitin® for the relief of symptoms of dry eye syndrome and cataract maintenance treatment without a prescription should consult a doctor in the following cases:
- use of the drug to relieve symptoms of dry eye syndrome for 6 weeks or more;
- the presence of purulent discharge from the eyes;
- redness of the areas of the eye covered by the eyelids;
- sudden (from one day to a month) deterioration of vision;
- the appearance of new symptoms or changes in previously observed symptoms affecting the organs of vision.
Patients suffering from recurring symptoms of dry eye or progressive vision loss due to cataracts for a long time should be regularly examined by an ophthalmologist.
Effect on the ability to drive vehicles and machinery If short-term blurred vision occurs after using the drug, it is not recommended to drive vehicles or engage in activities that require clear visual perception until it is restored.
Release form Eye drops with a concentration of 0.155 mcg/ml in polyethylene bottles of 5 ml or 10 ml with stoppers - droppers and screw caps. Each bottle with instructions for use is placed in a cardboard pack.
Storage conditions In a dark place at a temperature from +2 to +8 0C. Store the opened bottle in a place protected from light at a temperature from +2 to +8 0C; use within 1 month. Keep out of the reach of children!
Shelf life: 2 years. Do not use the drug after the expiration date indicated on the package.
Conditions of release Dispensed without a prescription.
Name, address of the manufacturer of the medicinal product and address of the place of production of the medicinal product 1. CJSC Framon, 121552, Moscow, st. 3rd Cherepkovskaya, 15a, building 7. 2. CJSC “Firn M”, 108804, Moscow, Kokoshkino settlement, settlement. Kokoshkino, st. Dzerzhinskogo, 4. Organization carrying out quality control: LLC "Research Institute of Mitoengineering of Moscow State University", 119992, Moscow, st. Leninskie Gory, vl. 1, page 73
Organization accepting complaints from consumers: Mitotech LLC, 119234, Moscow, st. Leninskie Gory, 1, building 77, floor 1, room 101A. Phone fax.
Side effects
The drug is usually well tolerated. Sometimes, after starting to instill eye drops, local negative reactions may develop:
- Brief burning sensation in the eyes.
- The appearance of a feeling of discomfort in the form of “sand in the eyes.”
- Redness of the conjunctiva.
Local reactions are usually reversible and disappear on their own after a short period of time, so discontinuation of the drug is not required. The development of local allergic reactions, which include skin rash, itching, swelling, and urticaria, cannot be ruled out.
Vizomitin's analogs
The unique ophthalmic composition does not have an identical replacement for the active compound. Any alternative that may be offered is chosen on the basis of the more traditional treatment of the diagnosis.
So, for “dry eye” and computer disorders, other drops can be used: Oftagel, Natural Tear, Optiv, Vidisik and others. Conservative therapy for cataracts is carried out only under the supervision of an ophthalmologist.
Important information for the development of a bacterial infection of the organ of vision - instructions for use of Vigamox eye drops.
special instructions
Before you start using the medication, it is important to pay attention to several special instructions:
- The eye drop procedure should be carried out carefully; hands must first be washed with soap.
- You should try not to touch the tip of the dropper bottle to your hands or other objects, as this greatly increases the risk of infection of the eye tissue.
- There is no data on the safety of the components of the drug for the body of a developing fetus and infant, therefore the use of the drug for pregnant and breastfeeding women is possible only after prescription by a doctor for strict medical reasons.
- Wearing contact lenses while using the medication is not recommended.
- The active and auxiliary components of the drug do not directly affect the functional state of the cerebral cortex and other structures of the central nervous system.
- After instillation, temporary blurred vision is possible, so you should not engage in potentially dangerous activities (driving a car) for 15 minutes.
Features of drug release
In addition to the main compound (its concentration is 0.155 mcg per 1 ml), the drops contain:
- benzalkonium chloride;
- hypromellose;
- water for injection and other substances.
The mixture appears as a clear or slightly colored liquid. The package contains instructions and a bottle with a 5 ml dropper. The medicine can be stored for 2 years from the date of release in the refrigerator (dark place at a temperature of 2–8 degrees). Once opened, the drops must be used within a month.
Tissue regeneration from the inside
You can find an analogue of Broxinac eye drops here.
Analogs
Optiv
An analogue of the drug that has similar therapeutic effects. The active ingredient is carmellose sodium. The drug has a moisturizing effect due to the formation of a film on the surface of the cornea and is prescribed for the complex treatment of dry eye syndrome in adults. Data on the possibility of using the drug in pediatric practice are currently limited. The possibility of use during pregnancy and breastfeeding is determined individually by the attending physician. An absolute contraindication is individual intolerance to any of the components of the drug.
Quinax
A medicine that is used for the conservative treatment of cataracts. It contains sodium azapentacene in the form of polysulfonate, which promotes the resorption of the opaque structures of the eye lens. The medicine is prescribed to adults. There is currently no sufficient data on the use of the drug in pediatric practice. The possibility of using the medicine for pregnant and breastfeeding women is determined by the attending physician individually according to strict indications, since there is no reliable data on safety for the body of a developing fetus or infant. An absolute contraindication for the use of the medication is hypersensitivity to the active or auxiliary components.
Aistil
The medication is a substitute for the drug Visomitin. It is available in the dosage form of eye drops and contains sodium hyaluronate, which is a naturally occurring polysaccharide, as the main active ingredient. The drug is used to moisturize cataracts during the treatment of dry eye syndrome. It is intended for adults. The use of the drug for children and adolescents under 18 years of age, as well as in the presence of individual intolerance to any of the components of the drug, is excluded. The possibility of use during pregnancy, as well as during breastfeeding, is determined individually by the attending physician.
The secret of success of biochemist Vladimir Petrovich Skulachev
Almost every cell in the body contains mitochondria - mini-power plants that can provide useful energy. A by-product of their work is reactive oxygen species, which in large quantities cause cell death. Antioxidants can combat this trend.
The development of the active substance is led by Vladimir Petrovich Skulachev, biochemist, Doctor of Science. Since 2005, his work has been aimed at studying the mechanisms of biological oxidation and the creation of geroprotectors (compounds that increase life expectancy).
Academician Skulachev
The active ingredient in eye drops has an unpronounceable chemical name - plastoquinonyldecyltriphenylphosphonium bromide, and therefore is usually referred to as SkQ1. It is these molecules that carry “Skulachev ions” that are able to prevent disastrous processes.
The world's first antioxidant, working at the level of mitochondria, affects the eyes in several directions at once:
- improves tear production;
- promotes epithelial renewal;
- stimulates metabolic processes;
- improves the quality of the tear film;
- protects cells from negative free radicals.
The study of the effect of the “Skulachev ion” on the body continues. Nothing can be confirmed yet, but research is underway on the effectiveness of the compound in age-related macular degeneration, i.e. disorders in the center of the retina. This disease is the leading cause of vision loss after age 50.
The complex effect of Visomitin allows it to be prescribed for dry eye syndrome; eye drops help against dryness and fatigue of the eyes, the consequences of intense computer loads, and even the initial stage of age-related cataracts. Extensive indications for use have now been tested in clinical trials, but research on the drug continues. The drug is also prescribed if there is pain in the eyes when the eyeball moves (until the cause is determined).
The innovative formula of the drug has no analogue in the world
The composition and basis of action of Brinzopt eye drops are described here.