Seretide is a combination bronchodilator indicated for the control of asthma. The drug reduces the risk of exacerbations by 30% and prevents the development of bronchospasm.
Using Seretide for asthma as part of basic therapy, it is possible to reduce the intensity of the inflammatory process and improve airway patency.
Composition and properties of Seretide
Seretide is a bronchodilator drug based on 2 bioactive compounds: salmeterol xinafoate and fluticasone propionate.
Salmeterol and fluticasone suppress bronchospasm and inflammation in the respiratory system. The active compounds stimulate β-adrenergic receptors in the respiratory tract. The main active ingredients have desensitizing, immunosuppressive, antishock and antitoxic effects.
The mechanism of action of the main components of the drug is different. Salmeterol prevents bronchospasm and relaxes their smooth muscles. The substance provides long-term bronchodilation.
A potent compound suppresses the negative effects of inflammatory mediators released by mast cells. It protects the body from the negative effects of histamine, leukotrienes and prostaglandin D2 for 12 hours. The response to inflammatory mediators decreases within 15-20 minutes after using Seretide.
Salmeterol suppresses the reaction to allergens even after its bronchodilator effect has ceased. Bronchial hyperreactivity decreases after the first inhalation. Therapeutic doses of the bioactive substance do not affect the activity of the cardiovascular system.
Fluticasone propionate restores lung function. The substance belongs to the category of glucocorticosteroids (GCS) - hormones that relieve inflammation and allergic reactions in the respiratory system. Fluticasone softens asthma symptoms and reduces the number of exacerbations. After using the medication, there are no adverse reactions characteristic of systemic corticosteroids.
With long-term use of Seretide in the maximum permitted dosage, the daily production of adrenal hormones does not exceed the maximum permissible norm in people of any age. If a person switches to fluticasone propionate from other inhaled corticosteroids, the production of adrenal hormones is stabilized.
Inhalations normalize the functioning of the adrenal glands. In response to the stimulating effect of fluticasone propionate, cortisol is synthesized in an acceptable concentration.
Seretide is an alternative treatment for people taking a β2-adrenergic agonist and inhaled corticosteroids together. The use of this medicine provides a greater therapeutic effect than monotherapy with corticosteroids.
For patients using salmeterol and fluticasone, reduce the dosage of fast-acting bronchodilators. Seretide reduces the incidence of exacerbations and asthma attacks. The quality of life of patients improves significantly.
Drug interactions
The use of non-selective and selective beta-blockers is indicated only in cases of urgent need; due to the risk of developing bronchospasm, such combinations are recommended to be avoided when treating patients with Seretide.
With simultaneous use of Seretide:
- monoamine oxidase inhibitors, tricyclic antidepressants - increase the risk of developing undesirable effects from the cardiovascular system;
- xanthine derivatives, diuretics and corticosteroids – contribute to the development of hypokalemia, especially in patients with hypoxia and exacerbation of bronchial asthma.
Since inhalation of fluticasone propionate does not cause an increase in its concentration in plasma due to high systemic clearance in the intestine and liver under the influence of the CYP3A4 isoenzyme of the cytochrome P450 system and intensive metabolism, the risk of developing clinically significant interactions with other drugs is negligible.
Ritonavir (a highly active inhibitor of the CYP3A4 isoenzyme) can cause a sharp increase in the plasma concentration of fluticasone propionate, and as a result, a significant decrease in serum cortisol levels. Intranasal or inhaled use of fluticasone propionate in combination with ritonavir may cause adrenal suppression and Cushing's syndrome. Therefore, simultaneous administration of these drugs should be avoided, except in cases where the risk of systemic side effects of GCS is outweighed by the potential benefits of therapy for the patient.
Other inhibitors of the CYP3A4 isoenzyme do not cause a significant increase in plasma fluticasone propionate concentrations and their use has virtually no effect on serum cortisol levels. However, strong CYP3A4 inhibitors such as ketoconazole are recommended to be used with caution as their use increases the risk of increased systemic drug effects.
The drug is compatible with cromoglycic acid.
Release form
Pharmaceutical companies produce medications in 3 versions:
- Seretide Evohaler is an inhalation aerosol, packaged in aluminum cans with a dispenser attachment. The container contains 120 doses of medicine. The bottle is packed in a cardboard box.
- Seretide Discus is a dosed powder preparation. Each blister contains 60 doses. The cardboard package contains the device necessary for inhalation, instructions and 1 blister.
- Seretide Multidisk is an inhalation powder poured into round plastic containers with a dispenser. Inhaler discs contain 28 or 60 doses of medication.
Reviews about Seretide
People who have practiced the treatment of bronchial asthma with the drug Seretide Evohaler or other varieties of this drug leave feedback on the long-term and successful use of the drug. People writing reviews on the forum note that there are practically no side effects. Leaving reviews about Seretide Multidisk and others, their authors note that after using the drug it is advisable to always rinse the mouth to avoid candidiasis . It is important to take Seretide only according to the regimen prescribed by your doctor and use it regularly.
Indications for use
Seretide is prescribed for the treatment of asthma if the patient requires the systematic use of inhaled corticosteroids and beta-agonists that act for a long time.
The medication is prescribed:
- in case of insufficient control of the disease with monotherapy with inhaled corticosteroids and irregular use of short-acting beta-agonists;
- patients who use long-acting beta2-adrenergic agonists and inhaled corticosteroids to control pathology;
- patients with persistent bronchial asthma in order to support and control the disease at the initial stage of treatment (with daily use of fast-acting drugs to relieve bronchospasm);
- people with obstructive pulmonary diseases for the purpose of maintenance treatment;
- patients with frequent exacerbations, persistent symptoms of pathology with constant treatment with bronchodilators.
Seretide™ overdose, symptoms and treatment
Symptoms of salmeterol overdose that can be expected are typical of excessive β2-adrenergic receptor stimulation and include tremor, headache, tachycardia, increased systolic blood pressure and hypokalemia. Cardioselective beta-adrenergic blockers are used as optimal antidotes, which should be used with caution when treating patients with a history of bronchospasm. If treatment with Seretide must be discontinued due to an overdose of the β2-agonist included in the drug, the patient should be prescribed appropriate corticosteroid replacement therapy. Inhalation of fluticasone propionate in doses higher than recommended may lead to temporary depression of the hypothalamic-pituitary-adrenal axis. This condition does not require immediate attention as adrenal function returns within a few days. However, when doses higher than recommended are used over a long period of time, significant suppression of adrenal function is possible. Cases of acute adrenal crises have been reported rarely, mainly in children receiving higher than recommended doses of the drug over a long period (several months or years). Hypoglycemia may occur, accompanied by impaired consciousness and/or convulsions. Factors that trigger acute adrenal crisis include trauma, surgery, infection, or a sharp reduction in the dose of inhaled fluticasone propionate. Therefore, Seretide should not be used in doses higher than recommended. The dosage regimen should be regularly reviewed and the dose reduced to the minimum level sufficient to effectively control the disease.
Contraindications
Seretide is not prescribed:
- patients with hypersensitivity to active substances and auxiliary components;
- patients in serious condition;
- children under 4 years old.
The medicine is used cautiously for the following concomitant pathologies:
- tuberculosis;
- diseases of the heart and blood vessels;
- diabetes mellitus;
- hypokalemia;
- diseases of the respiratory system caused by viruses, bacteria or fungi;
- diseases of the thyroid gland;
- pheochromocytoma;
- hypertension;
- arrhythmias;
- ischemia;
- cataracts;
- glaucoma;
- osteoporosis.
During pregnancy and lactation, Seretide is prescribed when the expected therapeutic effect is higher than the likely complications for the mother and baby.
Impact of the product
The instructions for use describe Seretide as a combination drug. Both leading components are responsible for different functions. The role of salmeterol is to prevent the occurrence of bronchospasm.
Fluticasone propionate is aimed at relieving inflammation, improving lung function, and also preventing exacerbation of the disease. It is also characterized by an antiallergic effect. The substance helps relieve symptoms. Thanks to it, exacerbations of the disease are observed much less frequently.
An additional merit of the drug is that it allows you to restore the body's response to bronchodilators. Because of this, the frequency of use of the latter is significantly reduced.
If the recommended dosages are followed and contraindications are taken into account, no pronounced side effects are observed. But you should not completely exclude the possibility of their manifestation, since it contains potent substances. The same applies to cases of overdose.
The most common side effects are:
- headache;
- cardiopalmus;
- arrhythmias (including extrasystole, tachycardia, etc.);
- nervousness;
- nausea and vomiting;
- skin rash;
- peripheral and angioedema;
- muscle spasms, etc.
With hypersensitivity to the medication or its individual component, some complained of disruptions in the perception of tastes, changes in voice, and even candidiasis of the pharynx or oral cavity. Rarely, even respiratory distress could be observed. The drug begins to act 20 minutes after its use.
Mode of application
A pronounced therapeutic effect is observed with regular use of the inhalation agent. The medicine is continued to be taken even after the symptoms of asthma disappear. The course of treatment and dosage is determined by the doctor. Patients are prescribed a dose in accordance with the severity of the disease.
When asthma cannot be controlled with glucocorticosteroid monotherapy, Seretide, a combination asthma medication, is prescribed.
Switching to a long-acting drug with a controlled course of the disease allows you to reduce the frequency of use of fast-acting corticosteroids.
Aerosol
Seretide Evohaler is a product for inhalation use. The aerosol is used daily, even in the absence of asthma attacks. The doctor monitors the medication intake and, if necessary, reduces the dosage of the drug. You cannot adjust your medication intake on your own.
If a beta2-receptor agonist is used simultaneously with the aerosol, the spray is inhaled once a day:
- for attacks that occur at night, take the medicine before bedtime;
- for attacks that occur during the day, the drug is used in the morning.
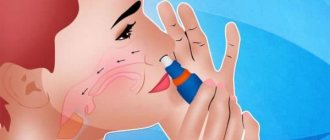
Algorithm for using the inhaler:
- When using for the first time or after a 7-day break, remove the mouthpiece cover and spray a fine cloud into the air.
- Before starting the procedure, the can must be shaken. When spraying, the device is held vertically.
- Take a deep breath and grab the nozzle with your lips. Release a fine cloud once while inhaling.
- Hold your breath.
- A 30-second interval is maintained before administering the second dose.
- After the procedure, the oral cavity is rinsed with water after a short period of time.
- Place a cap on the mouthpiece.
- The device is cleaned once every 7 days. The parts are wiped with a dry cloth.
- It is difficult for children to inhale on their own. Relatives should suggest how to spray the aerosol while inhaling.
The dose and duration of treatment is determined by the pulmonologist, taking into account the severity of asthma. Typically, adults and children over 12 years of age are recommended to take 2 inhalations daily in doses of 25 mcg/50 mcg, 25 mcg/125 mcg or 25 mcg/250 mcg. Children over 4 years of age are prescribed 1 dose of 25 mcg/50 mcg spray twice a day.
When asthma can be brought under control, the dosage is reduced to 1 procedure within 24 hours. The drug is withdrawn gradually. Abrupt cessation of treatment has a negative impact on the patient's condition.
Powder
Seretide Discus powder is used daily, regardless of the presence of asthmatic attacks. The duration of therapy and dosage are determined by the doctor.
Inhalation procedure:
- The plastic device is opened and charged.
- Then repeat actions similar to those performed when inhaling the spray.
- The number of unused doses is shown by the indicator.
Adults and children over 12 years of age take 1 serving (50 mcg/100 mcg, 50 mcg/250 mcg, 50 mcg/500 mcg) 2 times per 24 hours. For a short-term therapeutic course, 2 procedures are performed daily using 2 servings of Seretide Discus.
The duration of treatment should not exceed 14 days. Children from 4 years of age are given 1 serving (50 mcg/100 mcg) twice a day. The use of the medication is gradually abandoned.
Seretide Multidisc is a medicine intended for inhalation use only. The dosage and duration of the course are determined by the pulmonologist. Adults and children over 12 years of age undergo 2 procedures daily.
Patients receive 1 serving of the drug (50 mcg/100 mcg or 50 mcg/250 mcg or 50 mcg/500 mcg). If there are special indications, 2 portions are inhaled with each inhalation.
An increased dosage of the drug is prescribed for no more than 14 days. Children from 4 years of age are given 1 serving of the product (50 mcg/100 mcg). Carry out 2 inhalation procedures per day.
Seretide price, where to buy
The price of Seretide Multidisk 50 + 500 ranges from 2000 to 2100 rubles.
The price of Seretide Discus 25 + 250 is on average 2000 rubles; an inhaler 25 + 125 will cost approximately 1,500 rubles.
You can buy Seretide Evohaler at a price from 1800 to 3500 rubles, depending on the dosage.
- Online pharmacies in RussiaRussia
- Online pharmacies in UkraineUkraine
- Online pharmacies in KazakhstanKazakhstan
ZdravCity
- Seretide Multidisk powder for injection.
dosage 50 mcg+250 mcg/dose 60 dosesGlaxo Wellcome Production RUB 1,516 order - Seretide aerosol for in. dosage 25mcg+50mcg/dose 120 dosesGlaxo Wellcome Production
RUR 977 order
- Seretide aerosol for in. dosage 25mcg+125mcg/dose 120 dosesGlaxo Wellcome Production
RUB 1,324 order
- Seretide aerosol for in. dosage 25mcg+250mcg/dose 120 dosesGlaxo Wellcome Production
RUB 1,911 order
- Seretide Multidisk powder for injection. dosage 50 mcg+100 mcg/dose 60 dosesGlaxo Wellcome Production
RUB 1,076 order
Pharmacy Dialogue
- Seretide multidisc (vial 50mcg+250mcg 60 doses)Glaxo-Wellcome
RUB 1,368 order
- Seretide (aer. 25mcg+50mcg 120 doses)Glaxo-Wellcome
RUB 1,023 order
- Seretide multidisk (vial 50mcg+100mcg 60 doses)Glaxo Operations
RUB 1,099 order
- Seretide (aer. 25mcg+125mcg 120 doses)Glaxo-Wellcome
RUB 1,355 order
- Seretide multidisc (vial 50mcg+250mcg 60 doses)Glaxo Operations
RUB 1,364 order
show more
Pharmacy24
- Seretide Discus 250 mcg / 50 mcg N60 powder Glaxo Operations UK Limited, Great Britain / GlaxoWellcome Production, France
542 UAH. order - Seretide Evohaler 250 mcg 120 doses aerosol Glaxo Wellcome Production, France
693 UAH.order
- Seretide Evohaler 50 mcg 120 doses aerosol Glaxo Wellcome Production, France
354 UAH. order
- Seretide Discus 500 mcg/50 mcg N60 powder Glaxo Operations UK Limited, Great Britain / GlaxoWellcome Production, France
795 UAH. order
- Seretide Discus 100 mcg/ 50 mcg N60 powder Glaxo Operations UK Limited, Great Britain / GlaxoWellcome Production, France
400 UAH order
PaniPharmacy
- Seretide Evohaler aerosol Seretide Evohaler aerosol 25mcg/125mcg/dose 120 doses France, Glaxo Wellcome Production
524 UAH order
- Seretide Evohaler aerosol Seretide Evohaler aerosol 25mcg/50mcg/dose 120 doses France, Glaxo Wellcome Production
387 UAH. order
- Seretide discus Seretide Discus powder for inhalation 50mcg/500mcg/dose 60 doses UK, Glaxo Operations UK
848 UAH order
- Seretide Evohaler aerosol Seretide Evohaler aerosol 25mcg/250mcg/dose 120 doses France, Glaxo Wellcome Production
744 UAH order
show more
Side effects
When using Seretide, adverse reactions may occur. After using the inhaler you may experience:
- nausea and vomiting syndrome;
- dyspepsia;
- hyperglycemia;
- Cushing's syndrome;
- decreased mineralization of bone tissue;
- adrenal dysfunction;
- growth retardation in children;
- tachycardia and other cardiac pathologies;
- rhinonasopharyngitis, sinusitis;
- paradoxical bronchial spasm;
- pneumonia, bronchitis;
- urticaria, skin rashes, Quincke's edema, anaphylactic shock;
- cataract, glaucoma;
- muscle soreness and spasms;
- hoarseness of voice;
- joint pain;
- fungal infections in the oropharynx;
- side effects from the nervous system: irritability, anxiety, sleep disorders, headaches, hand tremors, behavioral disturbances.
In children, the likelihood of unwanted manifestations is quite high. It is necessary to constantly monitor the child’s condition.
Exceeding the 2-week dose of Seretide by 2 times leads to adverse reactions. Negative consequences develop under the influence of β-adrenergic agonists. Patients note:
- hand tremors;
- increased heart rate;
- convulsions;
- candidiasis of the oral mucosa;
- hoarseness of voice.
Excessive amounts of fluticasone suppress the adrenal glands. Organ dysfunction disappears spontaneously after discontinuation of the drug or reduction in dosage.
With a constant overdose, Cushing's syndrome occurs, growth retardation, and bone mineralization are impaired. Overdose can be avoided if the patient takes the medicine in recommended doses under the strict supervision of a doctor.
Seretide aerosol d/ing.dose.25mcg+125mcg/dose 120doses 2796
Description
Aerosol for inhalation metered Seretide®: inhaler - aluminum with a concave bottom, hermetically sealed with a metering valve; the internal surface of the inhaler and valve should not have visible defects. The contents of the inhaler are a white or almost white suspension. Powder for inhalation metered Seretide® Multidisc®: inhaler - a round plastic device of 2 shades of purple (dark purple and light purple) with a diameter of about 8.5 cm and a height of about 3 cm, with a dose counter showing 28 or 60 doses. The contents of the inhaler are white or almost white powder. When administered together by inhalation, salmeterol and fluticasone propionate do not affect each other’s pharmacokinetics, therefore the pharmacokinetic characteristics of each component of the drugs Seretide® and Seretide® Multidisc can be considered separately. Even despite the very low plasma concentrations of salmeterol and fluticasone propionate, interactions with other substrates and inhibitors of the CYP3A4 isoenzyme cannot be excluded. Salmeterol: acts locally in the lung tissue, so its plasma levels do not correlate with the therapeutic effect. Data on its pharmacokinetics are very limited due to technical problems: when inhaled in therapeutic doses, its Cmax in plasma is extremely low (about 200 pg/ml and below). After repeated inhalations of salmeterol xinafoate, hydroxynaphthoic acid can be detected in the blood, the Css of which is about 10 pg/ml. These concentrations are 1000 times lower than equilibrium levels observed in toxicity studies. Fluticasone propionate: The absolute bioavailability of inhaled fluticasone propionate in healthy subjects varies depending on the inhaler used (when using salmeterol/fluticasone propionate using a metered dose inhalation aerosol, it is 5.3% of the nominal dose). In patients with bronchial asthma and COPD, lower plasma concentrations of fluticasone propionate are observed. Systemic absorption occurs primarily through the lungs, and is initially faster but then slows down. Part of the inhalation dose may be swallowed, but this part makes a minimal contribution to systemic absorption due to the low solubility of the drug in water and due to its first-pass metabolism. Bioavailability from the gastrointestinal tract is less than 1%. As the inhalation dose increases, a linear increase in the plasma concentration of fluticasone propionate is observed. The distribution of fluticasone propionate is characterized by rapid plasma clearance (1150 ml/min), a large Vss (about 300 l) and a final half-life of approximately 8 hours. Fluticasone propionate has a relatively high degree of plasma protein binding (91%). It is rapidly eliminated from the blood, mainly as a result of metabolism under the action of the CYP3A4 isoenzyme to an inactive carboxyl metabolite. Renal clearance of unchanged fluticasone propionate is negligible (<0.2%), less than 5% of the dose is excreted in the urine as a metabolite. Caution must be exercised when using known CYP3A4 inhibitors and fluticasone propionate simultaneously, since in such situations the plasma levels of the latter may increase. It is excreted through the gastrointestinal tract, mainly in the form of a hydroxylated metabolite. The drugs Seretide® and Seretide® Multidisc are combination drugs containing salmeterol and fluticasone propionate, which have different mechanisms of action. Salmeterol prevents the occurrence of bronchospasm, fluticasone propionate improves pulmonary function and prevents exacerbations. The drugs may be an alternative for patients who simultaneously receive a β2-adrenergic agonist and inhaled corticosteroids. Salmeterol is a selective, long-acting (up to 12 hours) β2-adrenergic receptor agonist that has a long side chain that binds to the outer domain of the receptor. The pharmacological properties of salmeterol provide protection against histamine-induced bronchoconstriction and longer-lasting bronchodilation (lasting at least 12 hours) than short-acting β2-adrenergic receptor agonists. The onset of the bronchodilator effect is within 10–20 minutes. Salmeterol is a strong and long-acting inhibitor of the release of mast cell mediators such as histamine, LT and PG D2 from human lung tissue. Salmeterol inhibits the early and late phases of the response to inhaled allergens; the latter lasts more than 30 hours after administration of 1 dose, i.e. at a time when the bronchodilator effect is no longer present. A single administration of salmeterol weakens the hyperreactivity of the bronchial tree. This indicates that salmeterol, in addition to its bronchodilator activity, has an additional effect, the clinical significance of which has not been fully established. This mechanism of action differs from the anti-inflammatory effect of GCS. At therapeutic doses, salmeterol has no effect on CCC. Fluticasone propionate belongs to the group of corticosteroids for topical use and, when inhaled in recommended doses, has a pronounced anti-inflammatory and antiallergic effect in the lungs, which leads to a decrease in clinical symptoms and a decrease in the frequency of exacerbations of diseases accompanied by airway obstruction. Restores the patient's response to bronchodilators, allowing to reduce the frequency of their use. The effect of fluticasone propionate is not accompanied by adverse reactions characteristic of systemic corticosteroids. With long-term use of inhaled fluticasone propionate in the maximum recommended doses, the daily secretion of adrenal hormones remains within normal limits in both adults and children. After switching patients receiving other inhaled corticosteroids to fluticasone propionate, the daily secretion of adrenal hormones gradually improves, despite previous and current intermittent use of oral steroids. This indicates restoration of adrenal function with inhaled use of fluticasone propionate. With long-term use of fluticasone propionate, the reserve function of the adrenal cortex also remains within normal limits, as evidenced by the normal increase in cortisol production in response to appropriate stimulation (it must be taken into account that the residual decrease in adrenal reserve caused by previous therapy may persist for a long time). A study conducted among 318 adult patients with persistent bronchial asthma showed that when using a double dose of Seretide® and Seretide® Multidisc for 14 days (regardless of the dose of the components in the drug), there was a slight increase in the incidence of adverse events associated with the action of β- adrenergic agonists (tremor - 1 patient (1%), 0 patients - at the usual dose; rapid heartbeat - 6 patients (6%), 1 patient (<1%) - at the usual dose; convulsions - 6 patients (6%), 1 patient (<1%) - at the usual dose), while the frequency of adverse events associated with the action of the inhaled corticosteroid remains at the same level (for example, oral candidiasis - 6 patients (6%), 16 patients (8%) - at the usual dose ; hoarseness - 2 patients (2%), 4 patients (2%) - at the usual dose) compared to the usual treatment regimen. Thus, a double dose of the drug can be used in cases where patients require an additional short (up to 14 days) course of corticosteroid therapy. Treatment of bronchial asthma is recommended to be carried out in stages, monitoring the patient's clinical response to treatment and pulmonary function. The patient must be taught how to use the inhaler correctly. Seretide® and Seretide® Multidisc are not intended for the relief of acute symptoms, since in such cases a rapid and short-acting inhaled bronchodilator (for example salbutamol) should be used. Patients should be advised to always have medication available to relieve acute symptoms. Salmeterol/fluticasone propionate can be used for initial maintenance therapy in patients with persistent asthma (daily onset of symptoms or daily use of anti-attack medications) if there are indications for corticosteroids and their approximate dosage has been determined. More frequent use of short-acting bronchodilators to relieve symptoms indicates worsening disease control, and in such situations the patient should consult a doctor. Sudden and increasing deterioration in control of bronchospastic syndrome poses a potential threat to life, and in such situations the patient should also consult a doctor. It is possible that the doctor will prescribe a higher dose of GCS. If the dose of Seretide® and Seretide® Multidisk used does not provide adequate control of the disease, then the patient should also consult a doctor who may prescribe additional corticosteroids, and if an exacerbation is caused by an infection, then antibiotics. Due to the risk of exacerbation, treatment with Seretide® and Seretide® Multidisc should not be stopped abruptly; the dose of the drug should be reduced gradually under the supervision of a physician. Any inhaled GCS can cause systemic effects, especially with long-term use in high doses; It should be noted, however, that the likelihood of such symptoms occurring is much lower than with treatment with oral corticosteroids. Possible systemic effects include suppression of adrenal function, growth retardation in children and adolescents, decreased bone mineral density, and the development of cataracts and glaucoma. Considering the above, the dose of inhaled GCS should be titrated to the minimum that ensures the maintenance of effective control. In emergency and planned situations that can cause stress, it is always necessary to remember the possibility of adrenal suppression and be prepared to use GCS. When carrying out resuscitation measures or surgical interventions, it is necessary to determine the degree of adrenal insufficiency. It is recommended to regularly measure the height of children who receive long-term therapy with inhaled corticosteroids. Some patients may be more sensitive to the effects of inhaled corticosteroids than most patients. Due to the possibility of adrenal suppression, patients switched from oral corticosteroids to inhaled fluticasone propionate therapy should be treated with extreme caution and their adrenal function should be regularly monitored. When transferring patients from taking systemic corticosteroids to inhalation therapy, allergic reactions (for example, allergic rhinitis, eczema), which were previously suppressed by systemic corticosteroids, may occur. In such situations, it is recommended to carry out symptomatic treatment with antihistamines and/or topical drugs, incl. GCS for local use. After starting treatment with inhaled fluticasone propionate, systemic corticosteroids should be withdrawn gradually, and such patients should have a special card with them indicating the possible need for additional administration of corticosteroids in stressful situations. In patients with exacerbation of bronchial asthma, hypoxia, it is necessary to monitor the concentration of K+ in plasma. There are very rare reports of increased blood glucose levels, and this should be kept in mind when prescribing the combination of salmeterol with fluticasone propionate to patients with diabetes mellitus.
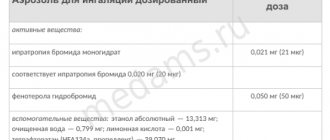
Compound
Seretide® Aerosol for inhalation dosed 1 dose salmeterol xinafoate 36.3 mcg (in terms of salmeterol 25 mcg) fluticasone propionate 50 mcg 125 mcg 250 mcg excipients: 1,1,1,2-tetrafluoroethane - up to 75 mg in an aluminum inhaler 120 doses (complete with dosing device); in a cardboard box 1 inhaler. Seretide® Multidisk® Powder for inhalation dosed 1 dose salmeterol xinafoate 72.5 mcg (in terms of salmeterol 50 mcg) fluticasone propionate (micronized) 100 mcg 250 mcg 500 mcg excipients: lactose monohydrate - up to 12.5 mg in a round plastic inhaler 1 blister with 28 or 60 cells; There is 1 inhaler in a cardboard pack.
Application
treatment of bronchial asthma in patients who are indicated for combination therapy with a long-acting β2-adrenergic agonist and an inhaled corticosteroid: - in patients with insufficient disease control on the background of constant monotherapy with inhaled corticosteroids with periodic use of a short-acting β2-agonist; - in patients with adequate disease control during therapy with an inhaled corticosteroid and a long-acting β2-adrenergic agonist; - as initial maintenance therapy in patients with persistent bronchial asthma (daily occurrence of symptoms, daily use of drugs for rapid relief of symptoms) if there are indications for prescribing GCS to achieve disease control; maintenance therapy for COPD in patients with a forced inspiratory volume (FEV1) <60% of the normal value (before inhalation of a bronchodilator) and a history of repeated exacerbations, in whom severe symptoms of the disease persist despite regular bronchodilator therapy. Pregnant and lactating women should prescribe the drug only if the expected benefit to the mother outweighs any possible risk to the fetus or child. Seretide® and Seretide® Multidisc contain salmeterol and fluticasone propionate, and therefore it should be expected that the drugs may cause side effects characteristic of these components. There is no evidence that the simultaneous use of salmeterol and fluticasone propionate causes additional side effects. May cause paradoxical bronchospasm. In this case, you should immediately use a short-acting inhaled bronchodilator, discontinue the drug, and begin alternative therapy if indicated. Salmeterol: pharmacological side effects of the beta2-adrenergic agonist have been described, such as tremor, palpitations and headache, hypokalemia, which, as a rule, are transient and weaken as salmeterol therapy is continued. In sensitive patients, arrhythmias (including atrial fibrillation, supraventricular tachycardia and premature beats) may occur. Arthralgia, nervousness, abdominal pain, nausea, vomiting and hypersensitivity reactions including skin rash, peripheral edema and angioedema have been reported. Cases of irritation of the mucous membranes of the oropharynx and changes in taste sensations (dysgeusia) have been described. Case reports of painful muscle spasms and very rare cases of hyperglycemia have been published. Fluticasone propionate: Some patients may experience a deepening or hoarseness of the voice and candidiasis (thrush) of the mouth and throat. Skin hypersensitivity reactions have been described. Hypersensitivity reactions have also been reported, manifested as angioedema (mainly swelling of the face and oropharynx), respiratory disorders (mainly shortness of breath and/or bronchospasm) and anaphylactic reactions. The severity and frequency of deepening of the voice and candidiasis can be reduced by rinsing the mouth with water after inhalation. Symptomatic candidiasis can be treated with topical antifungals while continuing therapy with Seretide® or Seretide® Multidisc. Very rarely, anxiety, sleep disturbances and behavioral disorders, including hyperactivity and irritability, have been reported (mainly in children); hyperglycemia. Systemic reactions are theoretically possible, including Cushing's syndrome or Cushingoid symptoms, adrenal suppression, growth retardation in children and adolescents, decreased bone mineral density, cataracts and glaucoma. With long-term use of doses of the combination of salmeterol with fluticasone propionate that exceed the permitted doses, significant depression of the function of the adrenal cortex is possible. There are very rare reports of acute adrenal crisis, which occurred primarily in children receiving higher than approved doses of this combination for a long time (several months or years); symptoms of adrenal crisis included hypoglycemia, accompanied by a decreased level of consciousness and/or convulsions. Due to the risk of developing bronchospasm, the simultaneous use of selective and non-selective beta-blockers should be avoided unless they are absolutely necessary for the patient. In normal situations, inhalation of fluticasone propionate is accompanied by low plasma concentrations due to intensive first-pass metabolism and high systemic clearance under the influence of the CYP3A4 isoenzyme of the cytochrome P450 system in the intestine and liver. This makes clinically significant interactions involving fluticasone propionate unlikely. Drug interaction studies have shown that ritonavir (a highly active inhibitor of the CYP3A4 isoenzyme) can cause a sharp increase in plasma concentrations of fluticasone propionate, resulting in a significant decrease in serum cortisol concentrations. There are reports of clinically significant drug interactions in patients who received fluticasone propionate and ritonavir concomitantly. These interactions have caused side effects such as Cushing's syndrome and adrenal suppression. Given the above, the simultaneous use of fluticasone propionate and ritonavir should be avoided, unless the potential benefit to the patient outweighs the risk of systemic side effects of GCS. Other inhibitors of the CYP3A4 isoenzyme cause a negligible (erythromycin) and insignificant (ketoconazole) increase in plasma fluticasone propionate levels, with virtually no decrease in serum cortisol concentrations. Despite this, caution is recommended when using fluticasone propionate concomitantly with strong CYP3A4 inhibitors (e.g. ketoconazole), since such combinations may increase the plasma concentrations of fluticasone propionate. Xanthine derivatives, corticosteroids and diuretics increase the risk of developing hypokalemia (especially in patients with exacerbation of bronchial asthma, during hypoxia). MAO inhibitors and tricyclic antidepressants increase the risk of side effects from the cardiovascular system. Compatible with cromoglycic acid. To obtain optimal effect, the drug should be used regularly, even in the absence of clinical symptoms of bronchial asthma and COPD. Determining the duration of the course of therapy and changing the dose of the drug is possible only on the recommendation of a doctor. The patient should be prescribed a dosage form of Seretide® or Seretide® Multidisk that contains a dose of fluticasone propionate appropriate to the severity of his disease. If a patient is unable to achieve adequate disease control with inhaled corticosteroid monotherapy, switching to combination therapy with salmeterol and fluticasone propionate at an equivalent corticosteroid dose may result in improved asthma control. For those patients in whom monotherapy with an inhaled corticosteroid provides adequate control of bronchial asthma, switching to inhaled therapy with a combination of salmeterol and fluticasone propionate may allow a reduction in the dose of the corticosteroid without loss of control of bronchial asthma. Seretide® Inhalation, intended for inhalation only. Recommended doses Adults and children 12 years of age and older: 2 inhalations (25 mcg salmeterol and 50 mcg fluticasone propionate) 2 times a day or 2 inhalations (25 mcg salmeterol and 125 mcg fluticasone propionate) 2 times a day, or 2 inhalations (25 mcg salmeterol and 250 mcg of fluticasone propionate) 2 times a day. Children 4 years and older: 2 inhalations (25 mcg salmeterol and 50 mcg fluticasone propionate) 2 times a day. Currently, there is no data on the use of Seretide® in children under 4 years of age. The dose of Seretide should be reduced to the lowest dose that provides effective control of symptoms. If control of symptoms is ensured by taking Seretide® 2 times a day, reducing the dose to the minimum effective may include a single dose of the drug per day. COPD For adult patients, the maximum recommended dose is 2 inhalations (25 mcg of salmeterol and 250 mcg of fluticasone propionate) 2 times a day. Special groups of patients There is no need to reduce the dose in elderly patients, as well as in patients with impaired renal or hepatic function. Seretide® Multidisc Inhalation, intended for inhalation only. Recommended doses Adults and children 12 years of age and older: 1 inhalation (50 mcg salmeterol and 100 mcg fluticasone propionate) 2 times a day or 1 inhalation (50 mcg salmeterol and 250 mcg fluticasone propionate) 2 times a day, or 1 inhalation (50 mcg salmeterol and 500 mcg of fluticasone propionate) 2 times a day. In adults over 18 years of age, doubling the dose while using any form of Seretide® Multidisk for up to 14 days maintains the same safety and tolerability as with regular use of this combination, 1 inhalation 2 times a day. The dose may be doubled in cases where patients require additional short-term (up to 14 days) inhaled corticosteroid therapy, as described in some asthma treatment guidelines. Children 4 years of age and older: 1 inhalation (50 mcg of salmeterol and 100 mcg of fluticasone propionate) 2 times a day. There is currently no data on the use of Seretide® Multidisk in children under 4 years of age. COPD For adult patients, the maximum recommended dose is 1 inhalation (50 mcg of salmeterol and 500 mcg of fluticasone propionate) 2 times a day. Special groups of patients There is no need to reduce the dose in elderly patients, as well as in patients with impaired renal or hepatic function. Seretide® Instructions for use of the inhaler Checking the inhaler: before using the inhaler for the first time or if the inhaler has not been used for a week or longer, you must remove the cap from the mouthpiece by lightly squeezing the cap on the sides, shake the inhaler well and release 1 stream into the air to make sure it works . Using the inhaler 1. Remove the cap from the mouthpiece by lightly squeezing the cap from the sides. 2. Inspect the inhaler inside and out, including the mouthpiece, for loose parts. 3. Shake the inhaler well to ensure that any loose parts are removed and that the contents of the inhaler are evenly mixed. 4. Hold the inhaler between your thumb and the other four fingers in a vertical position, bottom up, with your thumb resting on the base under the mouthpiece. 5. Exhale as deeply as possible, then place the mouthpiece in your mouth between your teeth, closing your lips around it without biting. 6. Immediately after you start inhaling through your mouth, press the top of the inhaler to spray Seretide® while continuing to inhale deeply and slowly. 7. While holding your breath, remove the inhaler from your mouth and remove your finger from the top of the inhaler. Continue holding your breath for as long as possible. 8. To carry out the second spray, hold the inhaler vertically and after about 30 s repeat steps 3–7. 9. After using the inhaler, rinse your mouth with water and then spit it out. 10. Close the mouthpiece cap by pressing and snapping into position. The drug can also be used through a spacer (for example Volumatic). Attention! When following steps 4, 5 and 6, you should not rush. You should begin inhaling as slowly as possible, just before pressing the inhaler valve. It is recommended to practice in front of a mirror the first few times. If you see a “fog” coming from the top of the inhaler or from the corners of your mouth, then you should start over from step 2. If your doctor has given different instructions for using the inhaler, then you should strictly follow them. You should contact your doctor if you have difficulty using your inhaler. Using the inhaler in children Young children cannot use the inhaler themselves and must be assisted by an adult. It is necessary to wait until the child exhales and activate the inhaler at the moment the child begins to inhale. You should practice using the inhaler with your child. Older children and adults with weak hands should hold the inhaler with both hands. In this case, both index fingers should be located on the top of the inhaler, and both thumbs should be on the base below the mouthpiece. For children, the drug is administered using an inhaler through a spacer with a face mask (for example, Babyhaler). Cleaning the inhaler: the inhaler must be cleaned at least once a week. Remove the protective cap from the mouthpiece. Do not remove the metal can from the plastic casing. Use a dry cloth or cotton swab to wipe the mouthpiece inside and out and the plastic casing outside. Close the mouthpiece with the protective cap. Do not immerse the metal can in water. Seretide® Multidisc Instructions for using the inhaler Inhaler device Multidisc is closed (Fig. 1) Multidisc is open (Fig. 2) The inhaler has an indicator that, after inhalation, shows the number of remaining doses. The numbers are in descending order from 60 to 0. Numbers from 5 to 0 are red, indicating that there are only a few doses left in the inhaler. The appearance of the number 0 in the window means that the inhaler is empty and unsuitable for further use. Using the inhaler To carry out inhalation, perform 4 sequential steps: 1) open the inhaler; 2) press the lever, 3) inhale the dose of the drug; 4) close the inhaler; 5) rinse your mouth with water. 1. Open the inhaler (Fig. 3). You should hold the body with one hand, placing the thumb of the other hand in a special recess. To open the inhaler, you need to press your thumb away from you until it clicks. 2. Press the lever (Fig. 4). You must hold the inhaler with the mouthpiece towards your face. The inhaler can be held with your right or left hand. Press the lever away from you until it stops until you hear a click. The inhaler is now ready for use. After pressing the lever, another cell with powder for inhalation is opened. In this case, the number of remaining doses decreases, which is indicated in the indicator window. You should press the lever only before inhalation, otherwise this will lead to wastage of the drug. 3. Inhale a dose of medicine (Fig. 5). You should hold the inhaler at some distance from your mouth and exhale deeply without force. Remember: never exhale into an inhaler! You need to wrap your lips tightly around the mouthpiece. Take a slow, deep breath through your mouth (not your nose). Remove the inhaler from your mouth. Hold your breath for about 10 seconds or longer as possible. Exhale slowly. Do not exhale into the inhaler. 4. Close the inhaler (Fig. 6) In order to close the inhaler, you need to place your thumb in a special recess and press towards you until it stops until you hear a click. The lever automatically returns to its original position. 5. After using the drug, rinse your mouth with water and spit it out. Cleaning the inhaler: after using the inhaler, the mouthpiece should be wiped with a dry cloth. Symptoms: objective and subjective symptoms of salmeterol overdose include tremor, headache and tachycardia. Inhalation of doses of fluticasone propionate higher than recommended may cause temporary depression of the hypothalamic-pituitary-adrenal axis. This usually does not require any emergency measures, since in most cases normal adrenal function is restored within a few days. With prolonged inhalation of excessively large doses of Seretide® and Seretide® Multidisc, significant adrenal suppression may occur. There are rare reports in the literature of acute adrenal crisis, which occurs predominantly in children receiving excessively high doses over a long period of time (several months or years); acute adrenal crisis is manifested by hypoglycemia, accompanied by confusion and/or convulsions. Situations that may trigger an acute adrenal crisis include trauma, surgery, infection, or a rapid reduction in the dose of fluticasone propionate. Treatment: antidotes are cardioselective β-blockers. In cases where it is necessary to discontinue the drugs Seretide® and Seretide® Multidisk due to an overdose of salmeterol included in its composition, the patient should be prescribed an appropriate replacement GCS. Patients should be aware that they should not take Seretide® and Seretide® Multidisk in doses higher than recommended. It is important to regularly assess the effectiveness of therapy and reduce the dose to the minimum effective, i.e. to one that provides effective control of disease symptoms. In case of chronic overdose, it is recommended to monitor the reserve function of the adrenal cortex. Increased sensitivity to the components of the drug; children under 4 years of age. With caution: pulmonary tuberculosis, fungal, viral or bacterial infections of the respiratory system, thyrotoxicosis, pheochromocytoma, diabetes mellitus, uncontrolled hypokalemia, idiopathic hypertrophic suboretal stenosis, uncontrolled hypertension, arrhythmia, lengthening of the QT interval on ECG, IBS, IBS, Hipox I am various genes, cataracts, glaucoma, hypothyroidism, osteoporosis, pregnancy, lactation.
Possible product names
- Seretide aeros. d/inhal. doses 25 mcg+125 mcg/dose 120 doses.
- SERETIDE 25 MCG/125 MCG AEROSOL 120 DOSES
- Seretide air. 0.25 mg/125 µg 120 doses
- SERETYD AIR. D/ING. 25MCG+125MCG/DOSE 120DOSES (11.08)
- SERETHIDE 25MCG+125MCG/DOSE AER.D/INHALATION DOSAGE. 120DOZ (R) $
- (Seretide) Seretide aerosol. d/inhal. doses 25 mcg+125 mcg/dose 120 doses.
special instructions
It is recommended to carry out therapy for bronchial asthma step by step. When carrying out treatment, be sure to monitor the development of the patient’s clinical reactions to the medication and the functioning of the respiratory system. Patients are taught how to use inhalation devices correctly.
Seretide is a long-acting drug for asthma; it is not used to quickly relieve asthma attacks and acute symptoms of the disease. For this purpose, short-acting inhaled bronchodilators are taken. People with asthma should keep a quick-acting asthma reliever (for example, Salbutamol) with them.
The medication is suitable for maintenance treatment in the early stages of pathology development. It is used for persistent bronchial asthma in 3 situations:
- for daily relief of symptoms of the disease;
- to prevent attacks that occur daily;
- if mandatory use of corticosteroids is required.
Chronic use of rapid-acting bronchodilators to suppress symptoms indicates the disease is getting out of control. If a person needs more of the drug, he should contact a pulmonologist.
The sudden release of bronchospasms out of control poses a threat to life. Increased shortness of breath after inhalation indicates the occurrence of paradoxical bronchospasm. The patient needs:
- urgently use a fast-acting bronchodilator;
- stop using Seretide;
- urgently consult a pulmonologist (the doctor may increase the dosage of glucocorticosteroids).
When the recommended dosage of Seretide does not allow to control the course of the pathology, the patient should consult with the attending physician. He will additionally prescribe GCS, and if a bacterial infection occurs, he will recommend antibiotics.
Discontinuation and side effects
It is strictly prohibited to stop taking Seretide; this practice leads to the development of dangerous complications. The dose of the drug should be reduced gradually under medical supervision.
The inhaled drug may cause unwanted systemic effects. Most often, negative manifestations occur with prolonged use of increased doses.
When using Seretide, side effects occur less frequently than when using oral corticosteroids. To minimize the risk of negative systemic effects, the dosage of the drug is reduced to the lower limit that allows you to control the disease.
The inhalation agent must be used in stressful situations in which the adrenal glands are inhibited. When carrying out resuscitation and operations, it is necessary to take into account the likelihood of developing adrenal insufficiency.
Children receiving additional inhaled corticosteroids have their height monitored regularly. The functioning of the adrenal glands must be examined:
- in patients with hypersensitivity to inhaled glucocorticosteroids;
- people who switched from oral use of corticosteroids to inhalation of Seretide.
In such patients, the functioning of the adrenal cortex is often disrupted, which causes the development of dangerous complications.
Allergic reactions
The transition from systemic corticosteroids to inhaled ones is sometimes accompanied by the appearance of an allergic reaction (rhinitis, eczema). Previously, undesirable effects were controlled by systemic glucocorticosteroids.
If allergies develop, symptomatic treatment is prescribed. Patients are prescribed:
- antihistamines;
- local medications, including corticosteroids.
After introducing Seretide into the treatment regimen, systemic corticosteroids are discontinued gradually. The patient is given a special card. The document emphasizes that a person under stress may require additional doses of glucocorticosteroids.
During exacerbation of the disease and oxygen starvation, the amount of potassium in the blood plasma is controlled. This helps prevent the development of hypokalemia. Seretide can increase blood sugar levels. People with diabetes need to monitor their glucose levels.
Selected Features and Compatibility
It has not yet been determined how Seretide affects the ability to drive a car and operate machinery. But doctors recommend taking into account the side effects that occur after using the product. Some negative manifestations (hand tremors, tachycardia) can provoke dangerous situations for the health and life of people.
Seretide aerosol is used in combination with beta2-blockers in exceptional cases due to possible bronchospasm. The combined use of drugs is allowed if there are serious reasons for such complex therapy.
The drug is not recommended to be combined with Ritonavir. Dangerous systemic effects may occur when medications are taken concomitantly. The therapeutic effect of the drug increases when it is used simultaneously with various beta-agonists.
Self-medication with Seretide is unacceptable. The drug may cause dangerous side effects. The medicine is used strictly as prescribed by the pulmonologist.
Pharmacodynamics and pharmacokinetics
Salmeterol has a local effect in lung tissue. During inhalation, plasma concentrations are low. After repeated inhalations, hydroxynaphthoic acid .
Fluticasone propionate has a bioavailability in a healthy person of 10-30% of the dose, depending on which inhaler is used.
People who suffer from bronchial asthma and COPD have lower concentrations of fluticasone propionate in their blood plasma. The drug is predominantly absorbed in the lungs; initially, more active adsorption is observed, later it slows down.
A person can swallow part of the dose used for inhalation, but its effect on the body is minimal, since fluticasone propionate is poorly soluble in water. When fluticasone propionate from the gastrointestinal tract, its bioavailability is less than 1%.
As inhalation doses increase, an increase in plasma concentrations of fluticasone propionate is observed. There is a high degree of binding to blood proteins of fluticasone propionate - 91%. Fluticasone propionate is actively eliminated from the blood mainly due to metabolism. The half-life is approximately 8 hours. Less than 5% of the dose received is excreted in the urine as a metabolite. It is excreted through the intestines mainly in the form of a hydroxylated metabolite .
Salmeterol almost does not enter the bloodstream when used in the form of inhalation. The substance does not accumulate in the body; 1 year after the start of therapy, the plasma level of salmeterol does not increase.
How to use Seretide multidisc
The instructions will tell you how to use Seretide Multidisc. The design is somewhat intricate, but it is quite easy to use. The inhaler has an indicator. It indicates the number of doses that are left.
The indicators are shown in reverse order from 28 or 60 to 0. The numerical interval 5–0 is colored red, which indicates a small remainder of doses. If the number 0 appears in the window, this means that there is nothing in the inhaler and it is no longer suitable.
The drug in the multidisk is contained in the form of a powder that must be inhaled. In order to do this, you need to take the following steps:
- Open Seretide inhaler. To do this, you need to place the body in one hand, and press the thumb of the other all the way into a special depression until a click occurs.
- Press the small lever. Holding the structure in one hand, you need to position the mouthpiece towards your mouth. With your free hand, you need to press the lever away from you until it clicks.
- Inhale the remedy. When the click is pressed, one dose of the drug is released. Therefore, before pressing, you should first exhale, and then, bringing it closer to your mouth, press and breathe.
- At the end of the procedure, the inhaler must be closed. In a certain depression, you need to press all the way towards yourself with your thumb. At this moment a click should also sound.
- Rinse your mouth. After closing the structure, you need to take clean room water into your mouth and rinse thoroughly, then spit it out. The mouthpiece must be wiped with a damp cloth.