Spreading
There are no reliable statistics on the incidence of amebiasis. When studying the infection rate of E. histolytica in the population of different countries, different data were obtained. This is due to a number of reasons and, in particular, to the fact that some authors classified the non-pathogenic E. hartmanni as E. histolytica. The average detection rate of E. histolytica is 10-15%. The vast majority of infected people are amoebone carriers. Clinically pronounced forms of amebiasis among patients with intestinal disorders in the temperate climate zone are 1-2%, and in Africa, South Asia, Central and South America - up to 20%. The possibility of epidemic outbreaks of amoebiasis is doubtful.
Diagnosis of amebiasis using serological tests
Due to the fact that with amoebiasis it is not always possible to identify amoebas in feces and aspirates, a serological study is carried out. Positive serological reactions are recorded in 75% of cases in patients with intestinal amebiasis, in 95% in patients with extraintestinal forms of amebiasis.
The indirect immunofluorescence reaction (IRIF) allows you to detect specific antibodies in the patient’s blood serum. A titer of 1:80 or more is diagnostic. In patients with amebiasis RNIF, the reaction is positive in 90 - 100% of cases. The reaction is negative in carriers of translucent (illuminated) forms. In patients with amoebic liver abscesses, RNIF is always positive and in high titers.
The indirect hemagglutination test (IRHA) is less informative, as it gives positive results not only in patients with amebiasis, but also in those previously infected.
Enzyme-linked immunosorbent assay (ELISA) is used to detect specific antibodies.
PCR (polymerase chain reaction) is the most promising technique in modern conditions. The high sensitivity of the test makes it possible to detect the DNA of parasites, even if there are only a few of them in the test material.
Etiology of amebiasis
The causative agent of amoebiasis is Entamoeba histolytica, dysenteric amoeba (synonym: Amoeba coli, Entamoeba dysenteriae, Entamoeba tetragena). Entamoeba histolytica belongs to the family Entamoebidae, order Amoebida, subclass Rhizopoda, class Sarcodina, phylum Protozoa. The causative agent of amebiasis was described in 1875 by the Russian scientist F. A. Lesh. Lesh experimentally proved the pathogenicity of the amoeba found in a patient with bloody diarrhea.
In the development cycle of E. histolytica there are two stages: the vegetative and the resting stage, or cysts. In the vegetative stage, the following forms are distinguished: tissue, large vegetative, luminal and precystic. Tissue and large vegetative forms are found in acute amoebiasis, luminal and precystic forms - during the period of convalescence and in cyst excretors.
Rice. 1. Entamoeba histolytica - vegetative stage: 1 - tissue form; 2 - large vegetative form; 3 — luminal form; 4 - precystic form
Fabric form
(Fig. 1, 1) is detected by histological examination of areas of affected tissue. Sometimes the tissue form can be found in intestinal discharge. The tissue form has a size of 20-25 microns. The cytoplasm is homogeneous without swallowed particles; along the periphery there is a narrow rim of ectoplasm. In fresh preparations, ectoplasmic pseudopodia and active forward movements can be observed. On hematoxylin-stained preparations, the cytoplasm is gray, the nucleus is in the form of a regular black ring with a small karyosome in the center.
Large vegetative form
(Fig. 1, 2). Size 30-60 microns. In a freshly isolated amoeba, the division of the cytoplasm into ecto- and endoplasm is clearly visible. In the latter, phagocytosed red blood cells may be visible; such amoebas are called erythrophages. In stained preparations, the morphological features of the cytoplasm and nucleus are similar to those of the tissue form. Red blood cells are stained black.
Translucent form
(Fig. 1, 3) can be found in the feces of cyst carriers after taking a laxative. Size 15-20 microns. The cytoplasm is finely vacuolated. In a calm state, the division of the cytoplasm into ecto- and endoplasm is not visible. When moving, it forms ectoplasmic pseudopodia. Does not phagocytose red blood cells. The structure of the nucleus is the same as that of the tissue form.
Precystic form
(Fig. 1, 4).
Size 15-20 microns. The cytoplasm is homogeneous without vacuoles and phagocytosed particles. In fresh amoebas, movement is weakly expressed. The core is the same as other forms. Rice.
2. Entamoeba histolytica - cysts (from top to bottom): 1, 2, 3 and 4-nucleated Cysts are found in patients during the period of convalescence or in cyst carriers. The shape is round, rarely oval. Size 9-14 microns. Depending on maturity, cysts have from 1 to 4 nuclei (Fig. 2). The structure of the nuclei is the same as that of vegetative forms. The cysts contain glycogen and chromatoid bodies, the latter in colored preparations looking like rods or black bars.
Symptoms of chronic intestinal amoebiasis
Chronic intestinal amebiasis occurs in a continuous or recurrent form. In the absence of antiparasitic treatment, the disease lasts about 10 years.
The recurrent course of the disease is characterized by periodic bloating and rumbling in the abdomen, upset stool, pain in the right lower abdomen, which is often mistaken for manifestations of appendicitis. During periods of exacerbations, the general condition remains satisfactory, body temperature does not rise.
With a continuous course of amebiasis, there is a gradual increase in symptoms such as abdominal pain, diarrhea alternating with constipation, stool frequency increases, blood appears in the stool, sometimes body temperature rises, and the liver gradually enlarges. Insufficient absorption of proteins and vitamins leads to exhaustion of the body and the development of asthenic syndrome. Hypochromic anemia develops. The patient loses weight. Appetite decreases, an unpleasant taste appears in the mouth, facial features become sharper. The tongue is thickly coated with a gray coating, the abdomen is retracted, heart sounds are muffled, and the heart rate increases.
Sigmoidoscopy reveals ulcers, cysts, polyps and amoebomas (inflammatory granulomas), which on radiographs show a filling defect, stimulating tumors. The development of scar tissue leads to stenosis (narrowing) of the intestine.
In 70% of cases, chronic intestinal amebiasis progresses slowly with subtle symptoms. Stool about 5 times a day, sometimes there is an admixture of mucus and blood. After some time, pain in the intestines appears.
In 30% of cases the disease becomes progressive. Diarrhea and cramping abdominal pain appear simultaneously. Already on the 2nd - 3rd day of the disease, mucus and blood appear in the stool. Body temperature is subfebrile. Insufficient intake of nutrients and vitamins into the body quickly leads the patient to asthenia and exhaustion.
Rice. 12. With the progressive course of the disease, asthenia and exhaustion of the patient quickly occur.
Epidemiology
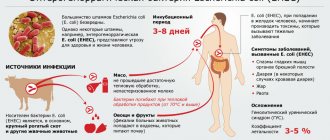
Amebiasis is widespread in areas with hot climates. The main source of infection in amebiasis is a person, who excretes up to 6 million amoeba cysts in 1 g of feces into the external environment with feces on some days. E. histolytica was occasionally found in monkeys, dogs, cats, pigs, cattle, and rats. Amoeba cysts in feces at a temperature of 13-17° remain viable for up to 15 days. On the soil surface in summer at a temperature of 10-50° they die after 2-3 days. In the intestines of flies, cysts are viable for 22 hours, in the feces of flies - 1 hour. Vegetative forms in feces degenerate within 15-20 minutes. Amoebiasis is contracted by ingesting amoebic cysts in contaminated food and water. Cysts are carried onto food by infected hands and flies.
Routes of infection
The spreader of amoebiasis is a sick person. It releases various types of amoebas and cysts into the external environment. Moreover, an infected person is contagious after completion of the acute phase of the disease. It can secrete amoebas for several years. The average number of amoebas that come out of a sick person per day is 9000 million. During the acute phase of amoebiasis, a person is not infectious, since he releases vegetative forms of amoebas into the external environment.
People become infected when cysts enter the body. Transmission occurs when eating unwashed food, or due to poor hygiene (dirty hand disease). In terms of infection, unwashed dishes, clothes, and bedding pose a danger. Cockroaches and flies can be carriers of infection.
Most often, men aged 20-50 years suffer from amoebiasis. After an infection, immunity is not developed. Amoebiasis is widespread in countries with humid and hot climates, although the infection occurs throughout the world.
Once in the intestine, the cyst transforms into a vegetative form and invades the intestinal wall. In it, it begins to produce substances that destroy organ tissue and lead to the formation of ulcerative defects. They appear from areas of erosion and abscesses, which are represented by nodules. When the nodule is destroyed, vegetative forms of amoebas emerge from it, and an ulcer appears in its place. The diameter of each area of ulceration can reach 25 mm.
Ulcers have the ability to merge. The more there are, the higher the likelihood of damage to the muscular layer of the intestine with its further perforation. This situation is life-threatening, as it leads to the development of peritonitis.
Damage to the vascular walls leads to bleeding of varying intensity. When the intestinal walls begin to heal, this can cause a narrowing of the lumen of the organ and its obstruction.
If amoebas penetrate the bloodstream, they are able to spread throughout the body, penetrating the liver, lungs, and brain. If the disease has become chronic, then there is a high probability of growth of a tumor-like amoeba in the intestinal lumen. It will be represented by granulation tissue and the body's own cells.
Pathological anatomy of amoebiasis
Rice.
3. Amoebas (in the upper corner on the left) in the submucosa, which is sharply swollen and poor in cellular elements, under the slightly changed mucous membrane of the colon (×220) Vegetative forms of Entamoeba histolytica cause edematous swelling in the thickness of the intestinal wall or in other organs (Fig. 3) and tissue necrosis. In places where amoebas are parasitized, the tissue loses its nuclei, becomes homogeneous and gradually melts. The cellular response to damage is very weak: only a few lymphocytes accumulate here and there or a slight proliferation of histiocytes occurs. A significant accumulation of leukocytes is a sign of a secondary bacterial infection, which often (especially in the intestines) complicates amoebiasis. A secondary infection (staphylococcal, streptococcal or caused by intestinal bacteria) accelerates the melting of tissues damaged by amoebas and gives the lesion a purulent, and in the case of an anaerobic infection, a gangrenous character. Amoebic lesions of different organs are generally similar, but their development and outcomes differ in some important features.
Intestinal amebiasis is the main form of the disease. Unlike bacterial dysentery, for which the primary localization of the inflammatory process in the mucous membrane of the distal part of the intestine is typical (although not obligatory), with amoebiasis lesions occur mainly in the cecum and ascending colon. The greatest changes (10-17.5%, according to different authors) are found in the colon, sigmoid and rectum. On sectional material (in severe and advanced forms of the disease), in almost half of the cases multiple lesions of the entire colon were observed.
With intestinal amebiasis, the earliest changes in the mucous membrane are detected in the form of a red spot with a yellowish-white dot in the middle. Subsequently, very characteristic ulcers develop, similar to round or oval plaques, reaching 1-2 cm in diameter and slightly rising above the surface of the almost unchanged mucous membrane. The edges of such lesions are moderately hyperemic, and in their central areas there is no mucous membrane. For a long time, the defect is made of necrotic tissue, disintegrated (as if hairy), colored greenish-gray, yellowish or dirty brown. Necrosis and swelling mainly affect the submucosal layer, spreading to the sides and under the remaining mucous membrane. Microscopic examination reveals amoebas at the border of tissue necrosis.
With amebiasis, lesions of varying ages are observed in the intestines. After the dead tissue melts, ulcers with undermined and overhanging edges form. They can heal almost without a trace. As the ulcers deepen, perforations are possible. On sectional material, perforations, sometimes multiple, are observed in 11 - 40% of patients with amebiasis. If the ulcer deepens slowly, perforation can be prevented by the development of adhesions to adjacent organs and peritoneum. Adhesions and scarring of ulcers occasionally lead to narrowing of the intestinal lumen. Even more rarely, excessive development of granulation tissue occurs with the formation of tumor-like thickenings of the intestinal wall - “amoebas”. There are no specific changes in the regional lymph nodes of the intestine during amebiasis (some researchers who described such changes mistook hyperplastic macrophages similar to them for amoebas). The spread of amoebas through the bloodstream occurs quite often. Metastatic foci occur predominantly in the liver.
Liver abscesses are found at autopsies in 36.5% of all cases of amebiasis. Essentially, these are not abscesses, but large foci of necrosis. They have an irregularly rounded shape, are gelatinous and yellowish or grayish in color.
When necrotic masses melt, cavities with semi-liquid dirty gray or brownish (due to blood) contents are formed. The remains of connective tissue layers give the inner surface of such cavities a hairy or sponge-like appearance. The necrosis zone is not delimited by anything for a long time, and individual lesions merge with each other. On their periphery one can find amoebas, which are rarely found in the contents of “abscesses”. Over time, a fibrous capsule forms, and small foci of necrosis completely scar under the influence of therapy. True ulcers form as a result of a secondary bacterial infection. E. coli and anaerobes give the pus a strong stench. Liver abscesses, single and multiple, are more often observed in the right lobe.
If left untreated, they reach very large sizes - up to the child’s head or more.
Quite rare complications of amebiasis include metastatic brain abscesses. They, as in the liver, are foci of necrosis and softening with a weak cellular reaction and amoebae at the border with preserved tissues.
Comparative electron microscopic studies have shown that trophozoites of E. histolytica cultured in vitro do not differ significantly from trophozoites obtained from the intestines of a sick person [El-Hashimi and Pittman (W. El-Hashimi, F. Pittman), 1970] or located in the liver tissue of artificially infected hamsters [Low and Megraith (S. Y. Lowe, V. G. Maegraith), 1970]. The first of the mentioned authors, without finding ultrastructural signs of extracellular secretion of enzymes by parasites, came to the conclusion that lytic enzymes, with which the pathogenic properties of E. histolytica are associated, are released only after the death of the amoebae.
Since 1965, reports have been published on primary amebic meningoencephalitis occurring in small epidemics in different countries, including European ones. These diseases, which quickly lead to death, are not caused by E. histolytica, but by free-living amoebas from the genus Naegleria. Infection occurs while swimming in pools. Amoebas penetrate the nasal mucosa, and then along the filaments of the olfactory nerve into the cranial cavity. The most severe destructive and inflammatory changes occur in the bulbus olfactorius, but also extend to the membranes of the brain (especially its base), in the superficial layers of which hemorrhagic necrosis is found. The inflammatory exudate in the membranes consists of polymorphonuclear leukocytes and macrophages, among which are amoebas, especially numerous in the perivascular spaces of small blood vessels.
Possible consequences and complications
Complications of the intestinal form of amebiasis are:
- perforation of the intestinal wall with the development of peritonitis is a complication characteristic of severe forms of the disease and is the cause of mortality in 20–45% of those who died from amoebiasis. Clinically manifested by the emergence and rapid increase in the intensity of the severity of the symptom complex of the acute abdomen;
- penetration of colon ulcers into other abdominal organs;
- pericolitis – recorded in 10% of patients with amebiasis. It is characterized by the development of adhesive fibrous peritonitis, most often in the area of the cecum or ascending colon. The main clinical sign of the disease is the formation of a painful infiltrate with a diameter of 3–15 cm, increased body temperature, and local tension in the muscles of the anterior abdominal wall. Pericolitis responds well to specific treatment and does not require surgical intervention;
- amoebic appendicitis - acute or chronic inflammation of the appendix. Surgical intervention in this case is undesirable, as it can provoke generalization of the invasion;
- intestinal obstruction - develops as a result of scar strictures of the colon, characterized by a clinical picture of low dynamic intestinal obstruction with a typical pain syndrome, palpable painful dense infiltrate, bloating and asymmetry of the abdomen;
- amoebic tumor (ameboma) is a rare complication of amebiasis. It is formed in the ascending or cecum, much less often in the splenic or hepatic flexures of the colon. It does not require surgical treatment, as it responds well to specific conservative therapy.
More rare complications of the intestinal form of amebiasis are prolapse of the rectal mucosa, colon polyposis, and intestinal bleeding.
Amebiasis most often affects children of older age groups and middle-aged people. In the overall structure of mortality from parasitic infections, it ranks second, second only to malaria.
The most dangerous complication of extraintestinal amebiasis is perforation of an amoebic abscess. A rupture of a hepatic amoebic abscess can occur in the subdiaphragmatic region limited by adhesions, the abdominal cavity, bile ducts, chest, subcutaneous or perinephric tissue. This complication is observed in 10–20% of cases of liver amoebiasis and is accompanied by a very high mortality rate (50–60%).
Pathogenesis
The pathogenesis of amebiasis is based on the penetration of amoebas into the wall and sometimes into the blood vessels of the intestine, which is determined by their mobility and the release of hyaluronidase and, possibly, other enzymes that lyse tissue. The penetration of amoebas is also facilitated by some bacteria that produce gnaluronidase. Ulcers occur in the intestines, and with hematogenous dissemination of amoebas, damage to the liver and other organs occurs. The patient's sensitization to amoeba allergens and autoallergens should also be taken into account. In a significant proportion of infected people, E. histolytica remains in the intestinal lumen and on the surface of its wall, without causing specific changes. These differences depend on the virulence of amoeba strains and the individual characteristics of people infected with amoebiasis. Clinically pronounced forms of amebiasis and its complications are more common in tropical and subtropical climates, which may be associated with overheating, disorders of water and salt metabolism, and dietary and lifestyle habits of the population.
general description
The causative agent of amebiasis is a histological or dysenteric amoeba, the habitat of which is the large intestine. It is noteworthy that in addition to this type of amoeba, a non-pathogenic type of amoeba is also often detected in this area.
The life cycle of a histological amoeba includes the vegetative (or trophozoite) as well as the cystic stage. Dysenteric amoeba, unlike other types of amoebas, has four forms of the vegetative stage in its life cycle. These include the tissue form, E. histolytica magna, the luminal form and the precystic form. Let's look briefly at each of them.
Clinical picture
Chronic intestinal amebiasis (rectal ampulla): 1 - intestinal lumen;
2- suppurating lymphoid follicles Chronic intestinal amebiasis (rectal ampulla): 1 - intestinal lumen; 2 - ulcer (in the center there is an overlay of pus, surrounded by a granulation zone; on the periphery - a hemorrhagic belt) Intestinal damage in amoebic dysentery Incubation period - from a week to several months. The disease begins acutely or with a prodromal period, when patients complain of malaise, weakness, decreased appetite, often abdominal pain, and headaches. Then diarrhea appears up to 5-15 times a day or more. The stool contains glassy mucus and often blood. The latter sometimes impregnates the mucus, and then the feces take on the appearance of raspberry jelly. In especially severe cases of amebiasis, usually when combined with bacterial dysentery, with extensive necrosis in the intestines, the stool acquires a fetid odor. Cramping abdominal pain, tenesmus, and burning sensation in the distal colon are common. The temperature is usually normal or low-grade. The tongue is coated with a whitish coating. The abdomen is swollen and painful on palpation in the iliac regions. The cecum is thickened and pasty, the sigmoid colon is spastically contracted. Sigmoidoscopy reveals swelling and focal hyperemia of the mucous membrane of the rectum and sigmoid colon, increased mucus production, erosion and often with undermined, raised edges of the ulcer, containing necrotic masses and surrounded by a belt of hyperemia (see colored figures). In the absence of specific treatment, diarrhea lasts from one to several weeks. The number of bowel movements gradually decreases, blood and later mucus disappear from the stool.
However, spontaneous recovery rarely occurs; usually the disease takes a chronic course with alternating periods of exacerbations and remissions. The clinical picture of exacerbations is similar to the manifestations of the acute form of vmebiasis. With a long course of chronic amoebiasis, asthenia, loss of nutrition, hypovitaminosis, and sometimes cachexia develop. There are also erased forms of amebiasis, in which minor intestinal disorders occur from time to time.
In children, acute amebiasis sometimes begins with an increase in temperature, which is usually not high; after 3-5, less often after 8 days, the fever stops. Pallor, drowsiness, lethargy, decreased appetite, nausea, less often vomiting, bloating, and bowel movements up to 10-15 times a day appear. The stool is initially mushy or liquid; in children of the first year of life it is green. After a few days, an admixture of mucus and blood appears in the stool. On palpation of the abdomen, pain and spasticity of the colon. If the sigmoid and rectum are affected - pain during defecation, tenesmus; In young children, the face turns red; they scream; Sometimes there is prolapse of the rectal mucosa. Anemia often occurs. Some patients have neutrophilic leukocytosis with a shift to the left; sometimes leukopenia. ROE is often accelerated. Total serum protein is reduced due to albumin. The pulse is rapid, heart sounds are muffled. Blood pressure is often reduced. Young children often develop bronchitis and pneumonia. In tropical countries, amebiasis in newborns sometimes initially occurs as toxic dyspepsia. The acute form of intestinal amebiasis usually lasts 2-3 weeks. The stool gradually returns to normal, mucus and blood disappear. However, in the absence of proper treatment, a relapse of the disease occurs after a few weeks, sometimes months and even years. With a prolonged course of amebiasis, schoolchildren’s performance decreases, weakness, lethargy sets in, dizziness, weight loss, abdominal pain, polyhypovitaminosis with a predominant deficiency of B complex vitamins appear.
Complications can be intestinal and extraintestinal. The first includes peritonitis, narrowing of the intestine, ameboma, etc., the second includes damage to the liver and other organs caused by the introduction of amoebae into them from the intestine through the hematogenous route. General and local peritonitis occurs as a result of perforation of the intestinal wall during an ulcerative-necrotic process or pericolitis. The symptomatology of peritonitis is usually the same as for perforated peritonitis of other etiologies (see Peritonitis), but often, especially when a large number of antibiotics are prescribed, it proceeds sluggishly, with mild pain. When scars form in the intestine at the site of deep ulcers, with adhesions in the abdominal cavity, and occasionally with an ameboma, relative obstruction occurs. Other intestinal complications include rectal prolapse, amoebic appendicitis, and intestinal bleeding.
Of the extraintestinal complications, the most common are amoebic hepatitis and liver abscess, which occur during acute intestinal A., after it, or several months or years later. Only in some patients in such cases the presence of amoebiasis in the past cannot be established. With amoebic hepatitis, the liver is enlarged, compacted, and its functions are impaired. Patients with amoebic liver abscess complain of pain in the right, less often the left, hypochondrium and epigastrium. Most patients experience fever - remitting or hectic, constant, irregular. Skin color is normal or pale. Jaundice caused by compression of the common bile duct by an abscess is rare. Patients walk carefully, bending towards the affected area. The liver is enlarged and painful. Sometimes it is possible to detect a perihepatic friction noise and the presence of a dome-shaped protrusion on the surface of the organ. X-ray examination reveals a high position of the right, and sometimes the left, dome of the diaphragm, and sometimes its local protrusion. With hepatolienography, the abscess appears against the background of the liver as a clearing with unclear contours. Sometimes dry or exudative pleurisy and basal pneumonia occur. With a large abscess of the left lobe of the liver - displacement of the heart. In the blood - neutrophilic leukocytosis with a shift to the left, occasionally a normal number of leukocytes and even leukopenia; ROE has been accelerated. With a long course of the disease - secondary hypochromic anemia.
There are acute, subacute and chronic forms of amoebic liver abscess. In the first case, there is high fever and severe intoxication. Death can occur within 1-2 weeks, less often later. In the subacute form, the violent course of the disease quickly gives way to a more benign one, the temperature drops, and intoxication decreases. Among the chronic forms of liver abscess, primary and secondary chronic are distinguished. In the first form, the painful process develops slowly, the temperature is normal or low-grade, abdominal pain is minor, and appetite is reduced. In the secondary chronic form, the initially acute course of the disease becomes sluggish and protracted. A liver abscess can open into the gallbladder, common bile duct, under the diaphragm, into the pleura, lungs, pericardium, abdominal cavity, stomach, right kidney, portal, inferior vena cava and hepatic veins.
Lung lesions also occur as a result of the penetration of amoebas into them from the intestine through the hematogenous route. There are two forms of pulmonary amebiasis - pneumopathy and abscess. With pneumopathy - chest pain, cough, dry or with scanty sputum, sometimes mixed with blood. The temperature is normal or low-grade. On percussion - dullness, on auscultation - subcrepitating rales, radiologically - darkening without signs of a cavity. ROE is accelerated, the number of leukocytes is normal or there is slight neutrophilic leukocytosis. The course is sluggish, in the absence of specific treatment - with transition to a lung abscess, which is characterized by a cough with copious chocolate-colored sputum, ulcerative laryngitis and tracheitis; X-ray - a cavity with a horizontal level of fluid, sometimes pleural empyema.
In the brain, when amoebas are introduced into it, an abscess occurs with focal and cerebral symptoms. Amoebic abscesses have been described in the spleen and other organs. Penetration of amoebas into the skin leads to ulcers and necrosis on the perineum, buttocks, at the site of rupture of liver and lung abscesses, near intestinal fistulas. Amebic lesions of the vagina and cervix have been described.
Diagnosis. Intestinal amebiasis must be differentiated from bacterial dysentery (see), balantidiasis (see), ulcerative colitis (see). In recognizing amoebic ulcerative colitis, the detection of the vegetative form of E. histolytica with phagocytosed erythrocytes in the feces is crucial, for which the preliminary administration of a laxative is sometimes necessary. In chronic, subacute and erased forms of amebic colitis and in parasite carriers, only cysts and pre-cystic forms of amoebas can be found; the former must be differentiated from smaller cysts of non-pathogenic E. hartmanni. If it is impossible to examine the feces on site, they are sent to the laboratory, mixing 1 g of them in 3 ml of a preservative of the following composition: 0.2% aqueous solution of sodium nitrate - 80 ml, formalin - 10 ml, Lugol's solution - 2 ml, glycerin - 2 ml.
Unlike bacterial dysentery, with amoebiasis there is usually no general intoxication, the temperature is normal or subfebrile, and lesions of the mucous membrane of the rectum and sigmoid colon are focal. In the stool with amebiasis there are macrophages, eosinophils, Charcot-Leyden crystals, and a scant number of neutrophils. Amebiasis and bacterial dysentery are often combined. Balantidiasis is recognized when the ciliate Balantidium coli is detected in the stool. With nonspecific ulcerative colitis - frequent intestinal bleeding, intermittent or hectic fever, feces have the appearance of minced meat, ROE up to 40-90 mm per hour.
When recognizing a liver abscess, data from palpation, X-ray examination, scanograms are taken into account, and sometimes they resort to puncture of the organ; the receipt of chocolate-colored pus indicates amoebiasis. An amoebic brain abscess is indicated by the presence of neurological symptoms and intestinal amebiasis in the present or past. Skin amoebiasis is characterized by localization of the process and ulceration with a necrotic rim. For all forms of amoebiasis, valuable data are obtained from serological reactions - precipitation in the gel and fluorescent antibodies.
Forecast
with timely recognition and treatment, favorable, it becomes serious with complications, but even in this case, the combination of specific therapy with surgical intervention, as a rule, saves the patient’s life.
Diagnostics
Photo: kiev-live.com
Today, in order to correctly diagnose a disease such as amoebiasis, it is necessary to undergo certain tests. For example:
- Stool analysis.
- Biopsy materials of ulcerative lesions.
- Selected rectal smears during sigmoidoscopy.
- Aspirate the contents of the liver abscess and so on.
And the most effective among them is a stool test for amoebiasis. Feces are examined under a microscope and vegetative forms are identified.
In some cases, it is necessary to take three to six tests for amoebiasis. This is done at the very first stages of the disease. This number of tests must be completed in order to finally determine whether the patient suffers from this disease. If a patient has certain symptoms, it is necessary to consult a doctor, who must make or refute a diagnosis.
Laboratory diagnostic procedure for amoebiasis
In order to diagnose amoebiasis correctly, it is necessary to conduct microscopy in physiological solution from freshly isolated feces of native smears, as well as smears that are pre-stained in Lugol's solution. If the disease is at an acute stage or at a pre-acute stage, then as a result of the study, specialists should detect the vegetative tissue form of the amoeba. If the patient’s carriers are asymptomatic, then a cyst and a small luminal form are detected in the laboratory. But the detection of luminal forms and cysts in feces is not a sufficient indication to make a diagnosis.
During laboratory diagnosis of amoebiasis, feces are used no later than fifteen minutes after defecation. This is a very important factor that must be taken into account, otherwise an incorrect diagnosis may be made.
There are also cases when specialists are not one hundred percent sure of the presence of effective signs of amoebiasis in a patient. And then they use the trichrome staining method for long-term storage of the preparations, and then send them for special examination to the reference laboratory.
Diagnosing intestinal amebiasis by examining freshly excreted feces under a microscope is the simplest and most convenient for doctors. In order to carry out such diagnostics, the clinic must be equipped with the latest modern equipment. But even the most experienced laboratory technician is not always able to assess the patient’s condition. And in such cases, it is precisely necessary to send the patient’s tests to more trained laboratories for further research and obtain a final assessment.
Differential diagnosis of amebiasis
In some cases, colonoscopy and rectoscopy are recommended. This is done when other tests indicate intestinal damage. If the doctor has ordered a colonoscopy and rectoscopy, a biopsy of the infected area of the intestine is taken from the patient, examined to identify amoebas, and then a diagnosis is made. Differential diagnosis of amebiasis helps to identify the presence of all kinds of ulcers and amoeba in the intestines. If, nevertheless, the patient has amebiasis, then the type of lesion will not be diffuse, but focal.
In order to diagnose extraintestinal amebiasis, as a rule, doctors prescribe computed tomography and ultrasonography to patients. Thanks to this, the number and quantity of abscesses in the patient’s body, their location are determined, and most importantly, the results of treatment are monitored.
It is also recommended to take an x-ray. It will help determine the number of abscesses in the lungs, whether there is effusion into the pleural cavity, as well as the condition of the diaphragm.
It should be noted that differential diagnosis is carried out in the case of diseases that are accompanied by hemocolitis. Dysenteric amoebiasis is characterized by a fairly short incubation period, very acute symptoms at the onset of the disease and minor clinical manifestations over a fairly short time period. And in this case, pathological changes in the blood progress very quickly. In addition, this disease tends to recur and is accompanied by manifestations of hemocolitis already in severe stages of the disease.
But the most important thing to remember in this case is the fact that the correct diagnosis can only be made by an experienced doctor who has a fairly high level of qualifications. You should never listen to the advice of ordinary people (friends, acquaintances, relatives). As soon as you experience the first signs and symptoms of a disease such as amoebiasis, you should immediately seek help from a specialist. And try to choose a doctor with a very good reputation. This is necessary to avoid misdiagnosis of the disease.
Treatment
Specific therapy is carried out mainly with emetine and metronidazole. Emetine is effective only for acute intestinal and extraintestinal amebiasis. Treatment is carried out only in a hospital setting. The drug is administered subcutaneously or intramuscularly in the form of a 1 or 2% solution. The daily dose for an adult (0.06 g, less often 0.08 g of the drug) is divided into two administrations. Daily doses for children: from 6 months to 1 year - 0.005 g; from 1 year to 2 years - 0.01 g; from 2 to 5 years - 0.015-0.02 g; from 5 to 9 years - 0.03 g; from 9 to 15 years - 0.04 g. Emetine is not prescribed for children under 6 months. The treatment cycle is 6-8 days, if necessary, it is repeated after 10-15 days. Possible side effects: vomiting, myalgia, neuritis, myocardial damage. Then the drug is discontinued. When the symptoms of acute intestinal amebiasis subside, quiniophone or antibiotics are prescribed. Quiniophone (synonym Yatren) is used orally 0.5 g three times a day for 5 days; after a break of 5 days - the second cycle of treatment. Daily doses for children: 1-2 years - 0.1 g; 2-3 years - 0.15 g; 3-4 years - 0.2 g; 4-5 years - 0.25 g; 5-6 years - 0.3 g; 6-8 years - 0.45 g; 8-12 years - 0.6 g; 12-13 years - 0.7 g; 13-15 years - 1.0 g; 16 years and older - 1.5 g. Monomycin is given orally for 5 days, 150,000 units four times a day; two cycles are performed with an interval of 5 days. Children under 5 years old - 10,000-25,000 units per 1 kg of weight per day, children 5 years and older - 100,000 units three to four times a day. Chlortetracycline (synonym biomycin) is prescribed orally at 0.2-0.3 g four times a day for 7-10 days; children under 3 years old - 25 mg per 1 kg of body weight per day in four doses, children over 3 years old - 0.075-0.1 g three to four times a day. Oxytetracycline (synonym terramycin) is used 0.2 g four times a day for 10 days; children under 3 years old - 25 mg per 1 kg of body weight per day, children over 3 years old 0.075-0.1 g three to four times a day.
Antibiotics do not have a direct amoebicidal effect, but they change the intestinal microflora and create unfavorable conditions for the life of amoebae.
Metronidazole (synonym: trichopolum, flagyl) is effective in all forms of amebiasis. The drug is prescribed 0.5 g three times a day for 3-5 days; for children, the daily dose is 25-30 mg per 1 kg of weight. For amoebic abscesses and skin amoebiasis, it is given 2.5 g per day for 1-3 days.
For extraintestinal amebiasis, the use of delagil (synonym: resoquine, hingamine, chloroquine) in combination with emetine is effective. The first drug is prescribed orally at 0.25 g twice a day for 2-3 weeks or 0.25 g three times a day for 7 days. Emetine in the form of a 1% solution is administered subcutaneously in a daily dose of 0.01 g (1 ml of solution) for 20 days. To suppress intestinal amebiasis during treatment with chloroquine and emetine, additional quiniophone or antibiotics are given. Large abscesses are opened or emptied by puncture, after which a liquid of the following composition is injected into the cavity: delagil 5% - 5 ml, penicillin - 500,000 units, streptomycin - 0.5 g, isotonic sodium chloride solution - 20 ml.
General strengthening therapy is important - a high-calorie diet, vitamins, iron, arsenic, autohemotherapy, etc. Diet: slimy soups, pureed porridges, steamed meat dishes, curdled milk, crackers, juices, strong tea, coffee.
In stubborn cases, climate change can give good results.
Symptoms of acute intestinal amoebiasis
The incubation period of intestinal amebiasis ranges from 1 week to 4 months or more. The disease begins with frequent bowel movements up to 6 times a day. Excessive feces mixed with mucus. There are no symptoms of intoxication in the initial period.
Gradually the patient's condition worsens. After 5-7 days, the fecal character of stool disappears. They are glassy mucus, in which blood appears over time. The stool takes on a “raspberry jelly” appearance. Signs of intoxication are increasing. Body temperature rises, appetite disappears, and the patient experiences nausea and vomiting. Bloating and pain in the lower abdomen, pain along the large intestine appear. The disease can affect different parts of the intestine - the cecosigmoid, rectal, and damage to the appendix.
An endoscopic examination reveals ulcers from 2 to 20 mm in diameter in the large intestine, located on the crests of the intestinal folds and in the deep layers of the mucous membrane. They have uneven, undermined edges. Brown necrotic masses are visible at the bottom. Each ulcer is surrounded by a “belt” of hyperemia. The surrounding mucous membrane is not changed.
Irrigoscopy reveals uneven filling of all parts of the large intestine, areas of spasm and rapid emptying.
Amebiasis in children begins acutely. Body temperature rises to 39°C. Nausea and vomiting appear. The child becomes sleepy. Stool up to 15 times a day, liquid or pasty. Dehydration of the body develops quickly.
The duration of the acute period is 4 - 6 weeks. Then a period of remission begins, lasting up to one month or more. Without adequate treatment, the disease recurs and becomes chronic over many years.
Rice. 10. Ulcers in amoebic dysentery on the tops of the ridges of the intestinal mucosa, 2 - foci of deep decay (photo on the right).
Prevention
A patient with amoebiasis must be hospitalized for the entire period of treatment. Staff of public catering establishments are subject to examination 2 times a year. If cysts or luminal forms of E. histolytica are detected in catering workers and children's institutions, sanitation is carried out with quiniophone without removal from work, and in case of intestinal disorder, treatment is carried out. It is necessary to observe public and personal hygiene measures. In hospitals, patients' feces are mixed with twice the volume of a 0.5% Lysol solution and the mixture is left to stand for 15-20 minutes. Measures to interrupt the transmission routes of amebiasis are the same as for other intestinal infections.
Forms of the disease
According to WHO recommendations adopted in 1970, the following forms of amebiasis are distinguished:
- intestinal;
- extraintestinal;
- cutaneous.
Russian infectious disease specialists regard the cutaneous and extraintestinal form of the disease as a complication of the intestinal form.
The most dangerous complication of extraintestinal amebiasis is perforation of an amoebic abscess. It is observed in 10–20% of cases of liver amoebiasis and is accompanied by a very high mortality rate (50–60%).
Intestinal amebiasis can occur in the form of acute or chronic (recurrent or continuous) processes of varying severity.
Amoebiasis is often recorded as a mixed infection, simultaneously with other protozoal and bacterial intestinal infections.
Bibliography
Amoebiasis, trans. from English, WHO, ser. tech. report, No. 421, Geneva, 1970; Gordon E.I. et al. Laboratory methods for studying pathogenic protozoa, M., 1957, bibliogr.; Yeolyan R. O. Liver abscesses and their treatment, M., 1949, bibliogr.; Kalinicheva I. G. Surgical complications of amebnos, Stalinabad, 1957, bibliogr.; Cashier with cue I.A. and Plotnikov N.N. Diseases of hot countries, p. 189, M., 1964, bibliogr.: Svanidze D.P. History of the study of amoebiasis and the fight against it in the USSR, M., 1955, bibliogr.; aka, Amebiasis and balantidiasis, M., 1959, bibliogr.; Soloviev M. M. and Pshenichny G. S. Study of sera from patients with intestinal amebiasis by the reaction of fluorescent antibodies, Med. parasitol., t. 40, no. 6, p. 643, 1971, bibliogr.; Schensnovich V.B. and Plotnikov N.N. Amoebic dysentery, Multivolume. manual, microbiol., wedge, and epidemiol. infectious diseases, ed. N. N. Zhukova-Verezhnikova, vol. 9, p. 160, M., 1968; El-Hashimi W. a. Pittman T. Ultrastructure of Entamoeba histolytica trophozoites obtained from the colon and from in vitro cultures, Amer. J. trop. Med. Hyg., v. 19, p. 215, 1970, bibliogr.; Lowe Ch. Y. a. Maegraith BG Electron microscopy of Entamoeba histolytica in host tissue, Ann. trop. Med. Parasit., v. 64, p. 469, 1970, bibliogr.; Manson-BahrP. E. a. Ormerd WE Amoebic and bacillary dysentery and the enteric fevers, Practitioner, v. 207, p. 154, 1971; Rubidge CJ, Scragg JN a. Powell SJ Treatment of children with acute amoebic dysentery, Arch. Dis. Childh., v. 45. p. 196, 1970, bibliogr.; Wilmot AJ Intestinal amoebiasis, Practitioner, v. 203, p. 634, 1969, bibliogr.
Etiology of Amebiasis
Matevosyan Sh. M. Parasitology and epidemiology of amebiasis, Yerevan, 1951, bibliogr.; Schensnovich V. B. The influence of cortisone and splenectomy on the reactivity of the body in experimental amebiasis, Med. parasitol., t. 30, no. 4, p. 455, 1961; Schensnovich V.B. and Soloviev M.M. Comparative virulence for rats of Entamoeba histolytica strains isolated from patients with amebiasis and from healthy carriers, ibid., No. 6, p. 694; Brumpt E. Precis de parasitologie, P., 1949; Craig CF Laboratory diagnosis of protozoan diseases, Philadelphia, 1942, bibliogr.; Deschiens R. L'amibiase et lamibe dysenterique, P., 1965, bibliogr.; Ho are CA Handbook of medical protozoology, L., 1949, bibliogr.
Symptoms of the fulminant form of intestinal amoebiasis
In 10% of cases, a fulminant form of amoebic dysentery is recorded, occurring with profuse diarrhea. Toxicosis and dehydration quickly develop, leading to exhaustion of the patient. Extensive ulcers appear in the intestines. The destruction of large vessels leads to bleeding. Intestinal perforation leads to the development of peritonitis. Liver abscess and amoebic hepatitis often develop.
The fulminant form of intestinal amebiasis develops in weakened and immunodeficient patients. Young children, elderly people, pregnant women and patients receiving hormonal therapy are susceptible to the disease.
Rice. 11. Endoscopic picture of intestinal amoebiasis.
General information about amoeba
There are two stages in the life cycle of amoeba - vegetative (trophozoite) and cystic. The vegetative stage has its own forms: tissue, erythrophage, luminal and precystic. Amoeba in tissue form has dimensions of 20 - 25 microns.
It can be detected only in acute amoebiasis and only in the affected tissues. It is found extremely rarely in feces. Amoebas in any form of the vegetative stage die very quickly in the external environment; they do not survive in feces for more than 30 minutes.
In acute amebiasis, they are found in the stool. A characteristic feature of this form is the ability to phagocytose (absorb) red blood cells. In addition, the protozoan in this form can secrete enzymes and penetrate the intestinal mucosa, forming ulcers.
The mechanism of transmission of amoebas is fecal-oral. This protozoan can enter the human body with water, vegetables and fruits, and food. The infection can be transmitted through household items and dirty hands. People become infected with amoebiasis very easily.
The cyst is the resting stage of the amoeba. It is a round formation with a diameter of about 15 microns with a double shell. This is a very stable stage; in feces it lasts up to 30 days, in tap water - up to 60, in waste water - up to 130, on the soil surface - up to 11, and at depth - up to 1 month. On human skin, cysts remain viable for about 5 minutes; under nails they live much longer - up to an hour.
Intestinal amoeba is widespread, but in countries with hot climates and poor social conditions it is easier to become infected. A clinically healthy amoeba carrier can excrete tens of millions of cysts in stool per day. There are about 480 million carriers of this microorganism in the world.
Amoeba cysts that enter the digestive tract are excysted in the small intestine under the influence of enzymes, and 8 trophozoites emerge from one destroyed cyst. They descend to the upper parts of the colon, where they become parasites. During their life, the amoebas again form cysts, which are excreted in the feces.
An intestinal amoeba can live in the intestines for a long time and not manifest itself in any way, feeding on bacteria and fungi there. However, under the influence of any factors (damage to the intestinal wall, inflammation, disturbances in peristalsis), it can turn into tissue form and begin to parasitize.
Amoebas secrete enzymes that melt the tissue of the intestinal walls and destroy its mucous layer. This leads to the formation of ulcers. The amoeba penetrates into the submucosal layer and multiplies there.
At the same time, the intestinal walls are restored, however, in the process, scars are formed, which can subsequently lead to intestinal stenosis. Also, during the scarring process, pseudopolyps can form.
Intestinal amebiasis is dangerous due to complications - intestinal perforation (most often in the area of the cecum), massive intestinal bleeding (due to erosions and large ulcers), amoebomas (tumor-like growths in the wall of the large intestine, consisting of fibroblasts, collagen, cellular elements and a small number of amoebae ), amoebic intestinal strictures that contribute to constipation and intestinal obstruction.
With the blood, amoebas can also enter the brain. In this case, single or multiple abscesses may develop in the brain, and the left hemisphere is most often affected. In this case, the disease ends in death.
Intestinal amoeba in the luminal form is isolated from those who have had acute amoebiasis or those suffering from a chronic form of this disease. This is a sluggishly moving protozoan up to 25 microns in size. The precystic form is a transitional form between the luminal stage of the vegetative form and the cyst. The amoeba is small, only 10-18 microns.
In some cases, amoebas can enter the bloodstream and spread to other organs. Most often, amoebas enter the liver, causing liver abscesses or amebic hepatitis.
This can happen both during acute amoebiasis and several months later. A liver abscess can cause unpleasant complications - peritonitis, damage to the organs of the chest cavity, including amoebic peritonitis and pleuropulmonary amebiasis.