Pharmacological properties of the drug Estrogel
Estrogen preparation for external use. Pharmacological features are determined by the presence of estradiol, which is a follicular hormone. During treatment with the drug, the severity of the manifestations of menopausal syndrome (including hot flashes, increased sweating, dystrophic changes in the vaginal mucosa, mood instability) significantly decreases. In women during menopause, the use of Estrogel prevents demineralization of bone tissue and the development of osteoporosis. Prevention of osteoporosis is individualized and proportional to the dose of available estrogen. When using 2.5 g of gel per day for 21 of 28 days, the effect is achieved in approximately 89% of women. The transdermal route of administration allows the use of natural estrogen produced by the ovaries, 17β-estradiol, for treatment. This pathway eliminates the effect of first pass through the liver, prevents stimulation of the synthesis of angiotensinogen, LDL and some coagulation factors, which reduces the risk of developing cardiovascular diseases, thromboembolism and metabolic disorders. When the gel is applied, the alcohol quickly evaporates and 17β-estradiol penetrates the skin, with most of it directly entering the systemic circulation, and a certain amount is retained in the subcutaneous fatty tissue and enters the systemic circulation gradually. The metabolism and excretion of 17β-estradiol when using Estrogel corresponds to the biotransformation and excretion of natural estrogens. The level of 17β-estradiol in the blood plasma of women during menopause when using 2.5 g of gel per day is 80 pg/ml. This corresponds to the normal estrone/estradiol ratio in women of childbearing age.
pharmachologic effect
Estrogen preparation for external use. Estradiol, the active substance of the drug Estrogel , is an estrogen.
Treatment with Estrogel significantly reduces the severity of menopausal syndrome (including hot flashes, increased sweating, vaginal dryness, low mood). The clinical effectiveness of Estrogel in the treatment of postmenopausal symptoms is comparable to that of oral estrogen medications.
The use of the drug helps reduce the concentration of total cholesterol without changing the cholesterol/HDL ratio.
The use of the drug inhibits the decrease in bone density during menopause.
It has a procoagulant effect, increases the synthesis of vitamin K-dependent blood coagulation factors (II, VII, IX, X) in the liver, reduces the concentration of antithrombin III.
Use of the drug Estrogel
Estrogel is prescribed in cycles - 24-28 days per month. Doses and duration of treatment are determined individually. One dose (2.5 g of gel) is applied once a day in the morning or evening to clean skin of the abdomen, lumbar region, thighs, shoulders or forearms in a thin layer until completely absorbed. If necessary, after 2-3 cycles, the dose can be adjusted depending on the clinical picture or estrogen deficiency. The gel should be applied in a thin layer to the surface of the skin (to the above areas). Massaging is not recommended. Avoid contact of the gel with the mammary glands and mucous membranes. Application is correct and effective if the gel is absorbed within 2–3 minutes. Mode of application. The gel is applied by the patient independently. To do this, you need to open the tube and pierce the metal membrane using a small punch located in the stopper. To calculate the daily dose, use a plastic dispenser spatula. It is necessary to press the tube over the ruler of the spatula, with 1 dose equal to a column (diameter of a pencil), the length of which coincides with the recess on the ruler. The recess has a dash, which makes it possible to divide the daily dose in half. One tube is designed for 30 doses. Apply the gel in a thin layer to the surface of the skin in the above areas. Should not be massaged. Prevent the gel from getting on the mammary glands and mucous membranes. Application is considered correct and effective if the gel is completely absorbed in less than 2–3 minutes.
Drug interactions
When used simultaneously, estrogens may reduce the effect of indirect anticoagulants, antihypertensive and hypoglycemic drugs (adjustment of the dosage regimen of hypoglycemic drugs may be required).
When used simultaneously, estrogens increase the effectiveness of lipid-lowering drugs.
With the simultaneous use of estrogens and drugs that induce microsomal liver enzymes (including barbiturates, carbamazepine, griseofulvin, rifampin), a decrease in the concentration of estradiol in the blood plasma is possible.
When used simultaneously, estrogens weaken the effect of male sex hormones and diuretic drugs.
When used simultaneously with estrogens, anxiolytic drugs (tranquilizers), opioid analgesics, and drugs for general anesthesia accelerate the metabolism of estradiol.
With the simultaneous use of estrogens with phenylbutazone and some antibiotics (including ampicillin, rifampicin), the concentration of estradiol in the blood plasma decreases, which is associated with changes in the microflora in the intestines.
When used simultaneously with estrogens, folic acid and thyroid hormone preparations enhance the effect of estradiol.
Side effects of the drug Estrogel
Most of the following undesirable effects have been observed with oral administration of artificial estrogens. When using Estrogel, side effects rarely occur, but it is recommended to stop using the drug if: cardiovascular failure, thromboembolic complications, cholestatic jaundice, benign mastopathy or uterine tumor (for example, an increase in the size of fibroids), liver adenoma (possible development of internal bleeding), galactorrhea ( pituitary adenoma must be excluded). Dose adjustment is required when signs of estrogen deficiency appear (hot flashes, frequent headaches, migraines, dry vaginal mucosa, eye irritation when using contact lenses) and signs of their high content (nausea, vomiting, abdominal pain, flatulence, engorgement of the mammary glands, irritability, peripheral edema, feeling of heaviness in the legs, increased secretion from the cervix). Possible uterine bleeding (the cause must be determined), exacerbation of epilepsy, chloasma or melanosis.
Side effect
From the side of the central nervous system:
headache, migraine, mood swings, dizziness.
From the reproductive system:
soreness, sensitivity and enlargement of the mammary glands, amenorrhea, breakthrough bleeding, menorrhagia, intermenstrual “spotting” vaginal discharge, swelling of the mammary glands, increased libido.
From the digestive system:
extremely rarely (because there is no “first pass” effect through the liver) - dyspepsia, spasms of smooth muscles of the gastrointestinal tract (including intestinal or biliary colic), epigastric pain, cholestatic jaundice, cholelithiasis, flatulence, anorexia, nausea, vomiting (mainly of central origin, mainly when using high doses), diarrhea, gallbladder obstruction, hepatitis, pancreatitis.
Local reactions:
irritation and hyperemia of the skin.
Other:
peripheral edema, changes in body weight, increased blood pressure, allergic skin reactions (urticaria, itching), thromboembolism, contact dermatitis, contact lens intolerance.
Side effects, as a rule, are mild, observed mainly in the first months of therapy and very rarely lead to discontinuation of the drug.
Special instructions for the use of the drug Estrogel
Since long-term use of estrogens in the form of monotherapy increases the risk of developing endometrial cancer, it is necessary to take a progestin drug for at least the last 12 days of each monthly cycle of treatment with Estrogel. Before starting treatment, it is necessary to conduct a complete clinical and gynecological examination. Periodic examinations should be carried out (including mammography) during drug therapy; their frequency and volume are determined individually. Particular attention should be paid to examining the mammary glands, uterus, measuring blood pressure and body weight, the state of the cardiovascular system and metabolism. Constant monitoring of the patient's condition is necessary in the following cases: ischemic stroke due to atherosclerosis, cerebral hemorrhage, retinal vein occlusion, obesity (due to the risk of developing venous thrombosis), bed rest and preparation for planned surgery (it is advisable to discontinue drug treatment 1 month before surgery), increased blood pressure. Careful monitoring is necessary for patients with endometriosis, uterine fibroids, endometrial hyperplasia, benign skin tumors, systemic lupus erythematosus, prolactin-secreting pituitary tumor, porphyria, with a history of recurrent cholestasis, renal failure, epilepsy, asthma, liver dysfunction, otospongiosis, a family history of history of breast cancer. Pregnancy and lactation: the use of the drug is not indicated. Women who took estrogens or estroprogestogens in the early stages of pregnancy do not need to undergo artificial termination of pregnancy.
Estrogel gel transderm. 0.06% 80g fl with dosing device
When treating postmenopausal symptoms, HRT should only be started if symptoms are present that adversely affect quality of life. A detailed assessment of the risks and benefits should be carried out at least once a year and HRT should only be prescribed if the benefits outweigh the risks. Data regarding the risks associated with HRT for the treatment of premature menopause are limited. However, given the low absolute risk of HRT in younger women, the benefit-risk ratio may be more favorable in these women than in older women.
Before starting or re-prescribing HRT, it is necessary to obtain a complete personal and family history. A medical examination should be conducted to identify possible contraindications and observe the necessary precautions when taking the drug (including examination of the pelvic organs and mammary glands).
During treatment, periodic examinations are recommended. The frequency and methods included in it are determined for each specific case individually. Tests, including mammography, should be carried out in accordance with accepted standards and adapted to the individual clinical needs of each individual case.
While the patient is taking HRT medications, a thorough assessment of all the benefits and risks of therapy should be carried out.
Conditions that require monitoring
If any of the following conditions are present, previously encountered and/or aggravated during pregnancy or previous hormonal therapy, the patient should be under constant medical supervision. It should be taken into account that these conditions may in rare cases recur or worsen during treatment with Estrogel®, in particular: uterine fibroids or endometriosis; risk factors for the development of thromboembolic diseases; risk factors for estrogen-dependent tumors (presence of first-degree relatives with breast cancer); arterial hypertension; liver diseases (for example, liver adenoma); diabetes mellitus with or without diabetic angiopathy; cholelithiasis; migraine and/or severe headache; systemic lupus erythematosus; history of endometrial hyperplasia; epilepsy; bronchial asthma; otosclerosis; hereditary angioedema.
Reasons for immediate discontinuation of therapy
Therapy should be discontinued if contraindications are found and/or in the following situations: jaundice or deterioration of liver function; marked increase in blood pressure; new attacks of migraine-like headache; pregnancy.
Hyperplasia and endometrial cancer
In women with an intact uterus, the risk of endometrial hyperplasia and cancer increases when taking estrogens for a long time. The risk of developing endometrial cancer in women using estrogen only is reported to be 2- to 12-fold higher than in women not using estrogen, depending on the duration of treatment and dose of estrogen. After stopping treatment, the increased risk may persist for at least 10 years.
The addition of a progestin in the last 12 days of the month/28 days of the cycle or continuous combined estrogen-progestin therapy in women with a non-removed uterus reduces the increased risk of endometrial hyperplasia and cancer associated with estrogen-only HRT.
During the first months of treatment, breakthrough bleeding and spotting may occur. If breakthrough bleeding or spotting appears after a period of treatment or continues after discontinuation of treatment, it is necessary to conduct an examination to identify the causes of their occurrence, including an endometrial biopsy to exclude endometrial malignancy.
The use of drugs for HRT containing only estrogen can lead to precancerous or malignant transformation of residual foci of endometriosis. Therefore, in women who have undergone hysterectomy for endometriosis, consideration should be given to adding a progestin to estrogen replacement therapy for the prevention of endometrial cancer if they are known to have residual endometriosis.
Mammary cancer
Available data suggest an increased risk of breast cancer in women receiving combined estrogen-progestin drugs and possibly also estrogen-only HRT drugs; this risk depends on the duration of HRT use.
The use of HRT drugs containing only estrogen
The WHI study found no increased risk of breast cancer in women who had a hysterectomy and used estrogen-only HRT. Observational studies in most cases report a small increase in the risk of diagnosing breast cancer, which is significantly lower than in women using combined estrogen-progestin drugs.
Use of combined estrogen-progestogen drugs for HRT The WHI study and epidemiological studies have found concurring data on an increased risk of breast cancer in women receiving combined estrogen-progestogen drugs for HRT; an increase in risk was detected after approximately 3 years of treatment.
The additional risk begins to appear after several years of treatment, but returns to baseline within a few (not more than five) years after stopping treatment.
HRT, in particular combined estrogen-progestin drugs, leads to increased density of mammographic images, which may interfere with radiological detection of breast cancer.
Ovarian cancer
Ovarian cancer is much less common than breast cancer. Long-term (at least 5-10 years) use of estrogen-only HRT drugs is associated with a slight increase in the risk of developing ovarian cancer. Some studies, including the WHI study, have found that long-term use of combination HRT drugs may carry a similar or less significant risk.
Venous thromboembolism
In women receiving HRT, there is an increase in the risk of developing VTE, in particular deep vein thrombosis or pulmonary embolism, compared with women who did not receive HRT, by 1.3-3 times. The likelihood of developing VTE is higher in the first year of HRT than in subsequent years.
Patients with known thrombophilic disorders may have an increased risk of developing VTE, and HRT may further increase this risk. Therefore, HRT is contraindicated in such patients.
The main risk factors for the development of VTE: individual or family history, estrogen use, old age, major surgery, prolonged immobilization, severe obesity (body mass index more than 30 kg/m2), pregnancy and the postpartum period, systemic lupus erythematosus, malignant neoplasms. There is no consensus on the possible role of varicose veins in the development of VTE.
The risk of VTE increases with prolonged immobilization, major trauma, or major surgery. Taking HRT medications should be stopped 4-6 weeks before planned abdominal surgery or orthopedic surgery on the lower extremities. Treatment can be resumed after complete restoration of motor ability.
In women who do not have a history of VTE but have first-degree relatives who experienced thrombosis at a young age, screening can be offered after a detailed discussion of its limitations (screening detects only some thrombophilic disorders). If the patient has a thrombophilic disorder that has been demonstrated by thrombosis in family members, or if there are “severe” defects (such as antithrombin deficiency, protein S or protein C deficiency, or a combination of defects), HRT is contraindicated.
In women already receiving chronic anticoagulant treatment, a careful assessment of the benefit-risk ratio of HRT is required.
If VTE develops after starting treatment, the drug should be discontinued. Patients should be instructed to contact their physician immediately if potential symptoms of thromboembolism (tenderness and/or swelling of the lower extremity, sudden chest pain, shortness of breath) occur.
Cardiac ischemia
Randomized controlled trials have not provided data on the preventive effect against myocardial infarction in women with or without coronary artery disease who received HRT with combined estrogen-progestin drugs or estrogen alone.
The use of HRT drugs containing only estrogen
There is no evidence from randomized controlled trials of an increased risk of coronary artery disease in patients who have undergone hysterectomy and are receiving estrogen-only HRT medications.
Use of combined estrogen-progestogen drugs for HRT When using combined estrogen-progestogen drugs for HRT, there is a slight increase in the relative risk of coronary artery disease. Since the initial absolute risk of CAD is largely dependent on age, the number of additional cases of CAD due to the use of estrogens in combination with progestins in healthy women approaching menopause is extremely small, but increases with age.
Ischemic stroke
HRT with combined estrogen-progestin drugs and estrogen alone is associated with an almost 1.5-fold increase in the risk of ischemic stroke. The relative risk does not change with age or with time since menopause. However, since baseline risk of stroke is largely dependent on age, the overall risk of stroke in women taking HRT will increase with age.
Other states
Estrogens cause fluid retention in the body. Patients with heart or kidney failure should be under constant medical supervision.
Careful monitoring is necessary when carrying out HRT in women with a history of hypertriglyceridemia, since in this condition, during estrogen therapy, rare cases of a sharp increase in the concentration of triglycerides in the blood plasma, leading to the development of pancreatitis, have been described.
Estrogens increase the concentration of thyroxine-binding globulin, leading to an increase in the total concentration of circulating thyroid hormones. The concentrations of free T3 and T4 do not change.
The content of other proteins, such as corticosteroid binding globulin and sex hormone binding globulin, may increase, which may lead to an increase in the total concentration of circulating corticosteroids and sex hormones. The concentrations of free or biologically active hormones do not change. It is also possible to increase the content of other blood plasma proteins (angiotensinogen (renin substrate), alpha-1-antitrypsin, ceruloplasmin).
Chloasma
In some cases, chloasma may develop, especially in women with a history of chloasma during pregnancy. Women who are prone to developing chloasma should minimize exposure to sunlight or ultraviolet radiation while using HRT.
Effect on cognitive function
HRT does not improve cognitive function. The WHI study showed a trend towards a possible increased risk of dementia in women who started long-term HRT with combined estrogen-progestin drugs or estrogen alone after the age of 65 years.
The use of Estrogel® should be carried out: by the woman herself, in the morning or evening, on clean skin.
Estrogel® does not leave stains.
Impact on the ability to drive vehicles and machinery
The effect of estrogens on the ability to drive vehicles and operate machinery has not been studied.
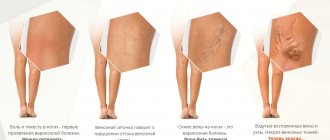
Symptoms of menopause
Below are the most common changes that a woman during menopause may experience::
- Tides. The scalp turns red and a feeling of heat appears, which lasts up to 5 minutes, mainly in the evening. Redness is also observed in the neck and chest area.
- Profuse sweating (especially at night).
- Heart rhythm disturbances, interruptions, heartbeats are strongly felt by the patient.
- Changes in the vaginal mucosa - it becomes thinner, dries out, becomes easily damaged, which leads to problems during sexual intercourse. Sexual desire disappears.
- Mood swings, irritability, unreasonable feelings of restlessness.
- Shortness of breath, drowsiness, weakness.
- Chills.
- Weight gain.
- Feeling of pain and itching in the bladder.
Positive effect of the product
Externally, improvements appear in the form of disappearance of unpleasant symptoms. Positive changes also occur in the body during treatment. Estrogel during menopause compensates for the lack of estrogen that occurs due to the fact that this hormone is produced in small quantities by the ovaries and adrenal glands during menopause.
Thanks to the restoration of thermoregulation functions, the activity of sweat secretion through the pores is reduced, and the dryness of the mucous membranes of the external genital organs is eliminated. There is less bad cholesterol in the blood, and the content of good cholesterol remains at an acceptable level. The psycho-emotional sphere also improves: the lady becomes calmer, worries less, her sleep normalizes and her resistance to stress increases.
Analogs
Estrogel has several complete analogues - medications that are identical in composition and therapeutic effects.
Dosage forms and their brief description:
- Klimara patch. The drug is made in Germany and contains estradiol. It has a feminizing and estrogenic effect.
- Menostar. Transdermal therapeutic system is prescribed for the prevention of osteoporosis. Can be used during menopause and for 5 years after its onset.
- Proginova. Dragee is prescribed for estrogen deficiency accompanied by vasomotor symptoms. Relieves manifestations of menopausal syndrome.
- Estrofem. Hormonal tablets for menopause containing estradiol. Manufacturer: Danish. The drug compensates for the lack of endogenous estrogens, normalizes protein and carbohydrate metabolism, restores water-salt balance, reduces cholesterol, increases libido, and protects against the development of osteoporosis.
- Estramone 50. Patch contains estradiol. Indicated for use as part of HRT during the peri- and postmenopausal period. Prevents the development of osteoporosis. Prescribed with caution to women over 65 years of age.
- Divigel. Finnish-made gel, according to some reviews, is better than Estrogel. Contains synthetic estradiol, identical to natural, is well absorbed and quickly absorbed into the blood. Indicated for menopausal syndrome.
Contraindications
Estrogel is contraindicated for the following conditions and diseases:
- the presence of malignant tumors or the risk of their formation;
- endometriosis;
- bleeding;
- thromboembolism;
- porphyria;
- predisposition to thrombosis, thromboembolism;
- liver disease, including a history of it;
- cholestatic itching, jaundice (diagnosed or experienced);
- pregnancy;
- lactation;
- hypersensitivity to the components of the gel.
Use with caution is recommended in the following situations:
- myoma;
- previous endometrial hyperplasia;
- risk of developing estrogen-dependent tumors;
- high blood pressure;
- cholelithiasis;
- headaches, migraines;
- diabetes;
- CHF, IHD;
- anemia;
- epilepsy;
- lupus erythematosus;
- pancreatitis;
- previous chloasma;
- predisposition to angioedema.
Estrogel is prescribed with caution to patients over 65 years of age. If the recommended dose is accidentally exceeded, nausea, vomiting, and bleeding may occur. In this case, stop use and consult a doctor for instructions.