Prohibited during pregnancy
Has restrictions when breastfeeding
Has restrictions for children
Can be taken by older people
Has limitations for liver problems
Has limitations for kidney problems
Lerivon is a medicine intended for the treatment of depressive conditions and some mental disorders of various etiologies. Treatment using the drug in most cases allows achieving positive results, however, in some cases, therapy using this medication is contraindicated and can lead to negative reactions of the body, therefore it is absolutely not suitable for self-medication. In particular, Lerivon, according to the instructions for use, is not recommended for impaired renal and liver function, as well as during pregnancy and pregnancy.
pharmachologic effect
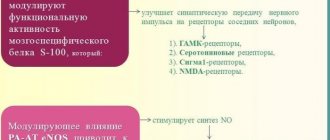
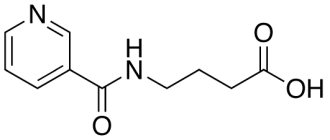
Mianserin , part of Lerivon, is a tetracyclic antidepressant. This is the so-called “second generation” of substances with antidepressant properties. The difference between mianserin and the first generation is the blockade of serotonin, histamine and alpha-adrenal receptors.
This range of actions makes Lerivon more like antipsychotics. However, at the same time, the drug blocks the reuptake of norepinephrine, increasing the concentration of this mediator. An increase in the amount of norepinephrine in the synapses of brain neurons helps improve mood and reduce anxiety.
In addition, Lerivon has low opioid activity, which also helps improve mood and reduce anxiety.
Effect of use
Mianserin is a drug that has an antidepressant effect and restores the normal state of the nervous system. This medicine belongs to the tetracyclic antidepressants (TCAs) and is a second generation antidepressant, therefore, Mianserin is sold without a prescription.
Mianserin, according to patients, has a milder effect on the nervous system and has a less pronounced effect as an antidepressant. This is its main difference from first generation antidepressants. Lerivon can be used by people suffering from disorders of the cardiovascular system, because it does not have a negative effect on its functioning. The drug also has a sedative and hypnotic effect.
Indications for use
Prescription of Lerivon is justified when the following diagnoses are established:
- A single depressive episode of any severity;
- Recurrent depression, during periods of exacerbation or remission;
- Bipolar disorder, current episode depressive;
- Organic pathology accompanied by depression;
- Anxiety-phobic disorder with a depressive component.
It is possible to take the drug in other cases if the attending physician considers it justified. For example, with subclinical depression or anxiety disorders.
Contraindications
The use of Lerivon is contraindicated in the following cases:
- Bipolar affective disorder, current manic episode; Mania or hypomania;
- Decompensated liver or kidney failure;
- Childhood;
- Pregnancy and feeding (no data on the effect on the fetus and baby);
- Individual intolerance to the main or auxiliary components of the drug.
Side effects
Common side effects that occur in more than one patient out of a hundred taking the drug include drowsiness, weight gain, swelling and tissue pastiness. Drowsiness is normal. It is due to the sedative effect of the medication. Occurs after starting treatment and stops after 1..2 months. It is worth considering that reducing the dosage does not lead to a decrease in drowsiness.
An increase in body weight is associated with a slowdown in metabolic processes in the body and a decrease in the influence of the sympathetic system on metabolism. Corrected by proper nutrition and exercise. Edema is associated with water retention in the body, which is a common side effect of tetracyclic antidepressants. Corrected by following the drinking regime.
Side effects such as decreased blood pressure, skin rashes not associated with an allergic reaction to the drug, and joint pain are much less common. Lerivon should be taken with caution by people prone to hypotension.
Less often than one patient per thousand taking side effects such as convulsions, extrapyramidal disorders, rhythm disturbances with a tendency to bradycardia and blockades are observed. In patients with bipolar affective disorder, in rare cases it provokes the transition of depression and hypomania.
It should be noted how Lerivon affects blood vessels.
Since the drug is both an alpha-adrenergic receptor antagonist and a norepinephrine agonist, it promotes peripheral vasodilation to a lesser extent than other antidepressants. However, short-term dilatation of peripheral vessels and a decrease in blood pressure are possible.
In addition, Lerivon has a negative effect on the immune system. In some patients, it causes a decrease in the granulocyte immune response, which leads to frequent bacterial infections.
Lerivon®
Use in children and adolescents under 18 years of age
Lerivon® should not be used in children and adolescents under 18 years of age. In clinical studies, suicidal behavior (suicidal attempts and suicidal ideation) and hostility (primarily aggression, oppositional behavior, and anger) were observed more frequently among children and adolescents treated with antidepressants compared with those receiving placebo.
If, based on clinical need, a decision is made to proceed with treatment, the patient should be closely monitored for the occurrence of suicidal symptoms. In addition, there are no long-term safety data in children and adolescents regarding growth, maturation, cognitive and behavioral development.
Suicide/suicidal thoughts or worsening clinical picture
Depression is characterized by an increased risk of suicidal ideation, self-harm and suicide (suicidal behavior). This risk persists until significant remission occurs. Since improvement may not occur within the first few weeks, patients should remain under the direct supervision of a specialist until improvement occurs. Accumulated clinical experience shows that the risk of suicide may increase in the early stages of recovery.
Patients with a history of suicidality or a high degree of suicidal ideation are at greater risk of suicidal ideation or suicide attempts and should be closely monitored during treatment. A meta-analysis of placebo-controlled clinical trials of antidepressants in adult patients with mental disorders found an increased risk of suicidal behavior in patients under 25 years of age taking antidepressants compared with patients receiving placebo. As a result, patients should be monitored very closely during treatment with antidepressants, especially those at high risk and in the early stages of treatment, and also after changes in the dosage regimen of the drug. Patients and their caregivers should be warned to monitor for any clinical signs of worsening, suicidal behavior or suicidal ideation, or unusual changes in behavior and to seek immediate medical attention if such symptoms occur.
Taking into account the likelihood of suicide, especially at the beginning of treatment, the patient should be given a limited number of Lerivon® tablets.
Bone marrow suppression has been reported to occur during treatment with Lerivon®, usually in the form of granulocytopenia or agranulocytosis. These reactions occurred most frequently after 4 to 6 weeks of treatment and were usually reversible when treatment was discontinued. They were observed in all age groups, but more often in elderly patients. If the patient develops fever, pharyngitis, stomatitis, or other signs of infection, treatment should be stopped and a complete blood count should be performed.
Lerivon®, like other antidepressants, may cause hypomania in susceptible subjects suffering from bipolar depressive illness. In this case, treatment with Lerivon® should be discontinued.
When treating patients with liver or kidney failure, heart disease, or diabetes mellitus, the usual precautions should be observed and the dose of concomitant therapy should be monitored.
During post-marketing surveillance of mianserin, QT prolongation and ventricular arrhythmias (including torsade de pointes) have been reported (see section "Side Effects"). Lerivon® should be used with caution in patients with risk factors for QT prolongation/TdP, including congenital long QT syndrome, age over 65 years, female gender, structural heart disease/left ventricular dysfunction, renal disease, or liver, simultaneous use of drugs that inhibit the metabolism of the drug Lerivon®, and simultaneous use of other drugs that prolong the QTc interval (see section “Interaction with other drugs”). Hypokalemia and hypomagnesemia should be corrected before starting treatment. Discontinuation of Lerivon® or reduction of its dose should be considered if the QTc interval is >500 ms or increases by more than 60 ms.
Patients with angle-closure glaucoma or symptoms of prostatic hyperplasia should be monitored due to the unpredictability of anticholinergic adverse events during treatment with Lerivon®.
If jaundice occurs, treatment with the drug should be discontinued.
If seizures occur, treatment with the drug should be discontinued.
Use in elderly patients
Based on limited clinical trial data, elderly patients were less susceptible to adverse reactions such as agitation, confusion, and postural hypotension with mianserin than with tricyclic or tetracyclic antidepressants, but any therapy with these drugs should be used with caution in this group of patients.
Hypomania
Lerivon®, like other antidepressants, may cause hypomania in susceptible subjects suffering from bipolar depressive illness. In this case, treatment with Lerivon® should be discontinued.
Surgical interventions
If surgery is necessary during mianserin therapy, the anesthetist should be informed of the treatment being performed.
Pheochromocytoma
Caution must be exercised when treating patients with pheochromocytoma.
Epilepsy
Regular and careful monitoring is necessary, as well as adherence to the dosage regimen in patients with epilepsy and organic brain damage syndrome. Clinical experience shows that epileptic seizures are rare during treatment with antidepressants. Lerivon® should be used with caution in patients with a history of seizures. Treatment should be discontinued if seizures develop or if the frequency of seizures increases.
Overdose
Symptoms of overdose can result from a significant increase in the dose of the drug, its uncontrolled use, as well as use together with alcohol or other antidepressants of the same group. All this leads to an increased chance of developing side effects.
In case of overdose, deep depression of consciousness, convulsive syndrome, symptoms of hypomania, depression of the respiratory center, persistent heart block, severe bradycardia and hypotension are possible. There is no antidote for Lerivon. It is necessary to rinse the stomach as quickly as possible, give sorbents, give an enema and take the patient to the intensive care unit.
Dosage regimen
Lerivon is started with an average therapeutic dose of 30 mg. Then, depending on the tolerability of the drug and its effectiveness in a particular patient, the dosage changes. The maximum permissible daily concentration of the active substance is 90 mg. As a rule, therapeutic doses do not exceed 60 mg. In elderly patients and patients with liver failure in the subcompensated stage, it is necessary to reduce the dosage by half.
The effectiveness of the drug should be assessed no earlier than 2 weeks from the start of therapy and only after that the dose of the medication taken should be increased. The second dosage increase should be no earlier than a month from the start of treatment. Further increase in dose is not advisable. If there is no effect, the drug should be changed.
Features of administration and dose
Lerivon is purchased to relieve symptoms of depression. The tablets are taken orally with water. They should not be broken or chewed when taken.
The drug Lerivon has a lot of positive reviews at an affordable price. For adult patients, the dosage of tablets is calculated individually. The initial daily dose is 30 mg. This dose should be increased over several days until the desired clinical effect appears. Usually the daily dosage is 60-90 mg; with this dose of Mianserin at a low price, the effect of taking the medicine is permanent.
Mianserin is also indicated for elderly people with depressive disorder at the price at the pharmacy. The initial dose for them should be no more than 30 mg. To get the desired effect, the dose can be increased once every few days. In this case, the maximum daily dose should be less than for adults.
If the daily dose of Mianserin, which was purchased to relieve symptoms of anxiety and depression, exceeds 30 mg, it can either be divided into several doses or taken at a time, but always at night.
A positive effect usually appears 2-4 weeks after starting to take the drug. If after 4 weeks of taking Lerivon at an affordable price there are no positive changes, the patient’s condition has not improved, the use must be stopped.
Instructions on how to take Lerivon
Lerivon is recommended to be taken at night, given its pronounced sedative effect. The drug can be taken once a day in the required dosage. Sometimes the daily dose is divided into two doses.
During the course of treatment, it is not recommended to drive a car or perform work that requires constant concentration. Lerivon does not affect suicide attempts and thoughts of suicide. Patients who cause harm to their health and life must undergo treatment under the supervision of a doctor in a hospital.
The average course of therapy is six months.
Drug interactions
Lerivon may enhance the depressant effect of alcohol on the central nervous system, and patients should be advised to abstain from drinking alcohol during treatment. Lerivon should not be used simultaneously with MAO inhibitors or within the next two weeks after the end of treatment with these drugs. Lerivon does not interact with betanidine, clonidine , methyldopa, guanethidine and propranolol (both in combination with and without hydralazine). However, it is recommended to monitor blood pressure in patients who are simultaneously treated with antihypertensive drugs. As with other antidepressants, Lerivon may interfere with the metabolism of coumarin derivatives, such as warfarin, which requires monitoring.
Analogs
Analogs of Lerivon are derivatives of mianserin or tolvon :
- Miaser;
- Velaxin;
- Melitor;
- Mirtel;
- Mirzaten;
- Deprim;
- Devaccinated;
- Vipax.
Despite the fact that all of the listed drugs have a similar medicinal substance, their dosages, side effects and contraindications may differ.
Purchasing the drug and its analogues
You can buy Lerivon in any large pharmacy or pharmaceutical markets, you can also order it online; the price of a package with 30 tablets in the Russian Federation at the end of 2021 ranges from 890 to 1300 rubles.
The drug should be stored in a dark place out of reach of children. The temperature should be no more than 30 degrees. The storage period is 5 years.
Analogues of Lerivon:
- Pyrazidol;
- Neuroplant;
- Velaxin;
- Venlaxor;
- Mirzaten;
- Mirtazapine Sandoz;
- Prefaxin;
- Miaser;
- Azafen;
- Melitor;
- Cymbalta;
- Deprim Forte;
- Remeron;
- Esprital 45.
Reviews
Anton G .: “My first episode of depression happened more than 20 years ago. Since then it has returned periodically, sometimes without leaving me for several years. During this time, I changed 4 antidepressants and settled on Lerivon. I take it at night, as recommended, 30 mg. The first week I felt sleepy all day, it was difficult to concentrate, but then everything went away. Within a month I noticed a significant improvement in my mood, and at the moment I continue taking it.”
Tatyana F .: “Lerivon was prescribed to me due to an anxiety-phobic disorder with mood swings. The drug worked for me, I didn’t even notice any side effects. For the first month I took 60 mg of it and couldn’t believe that such an effect could happen. Anxiety decreased, mood improved, there was not even any drowsiness. But after a month, a side effect arose - weight gain. I gained 5 kg. I plan to stop taking the drug because I can’t control my hunger and I’m afraid of gaining even more weight.”
Review from a psychiatrist : “Lerivon is a relatively new drug, its properties different from its predecessors. Its pleasant side is the fairly rapid onset of a visible effect and a small number of side effects. Most often we encounter the sedative properties of the drug, but they quickly pass if the patient does not refuse to take it for this reason. In our clinic, it is customary to prescribe Lerivon only to inpatients, in order to be able to adjust the dose.”
Content