Release form and composition
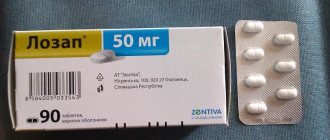
Lozap Plus is produced in the form of film-coated tablets: oblong, with separating marks on both sides, light yellow in color (10 pcs. in blisters, in a cardboard pack of 3, 6 or 9 blisters; 15 pcs. in blisters, in cardboard pack of 2, 4 or 6 blisters).
Composition per 1 tablet:
- active ingredients: losartan potassium – 50 mg; hydrochlorothiazide – 12.5 mg;
- auxiliary ingredients: microcrystalline cellulose, mannitol, croscarmellose sodium, magnesium stearate, povidone;
- film shell: hypromellose 2910/5, talc, macrogol 6000, simethicone emulsion, Crimson dye [Ponceau 4R] (E124), Quinoline yellow dye (E104).
Generics
The indications and contraindications for Lozap are now clear, but what to do if for some reason you cannot use this medicine? Look for analogues. On the modern pharmacological market there are quite a lot of drugs that are similar in their actions, so there will be no problems with the choice.
So, what medications can be called analogues?
- "Blocktran."
- "Vasotens".
- "Brozaar."
- "Vero-Losartan".
- "Cardomin-Sanovel".
- "Zisakar".
- "Kozaar."
- "Carzartan".
- "Lozarel."
- "Lakea."
- "Losartan."
- "Losartan McLeods."
- "Losartan potassium."
- "Losartan-Richter".
- "Lorista".
- "Losartan-Teva".
- "Losakon."
- "Renicard."
- "Presartan."
As you can see, if you wish, you can find a drug that has the same properties, but is free of side effects or contraindications.
Pharmacological properties
Pharmacodynamics
Losartan is a specific angiotensin II receptor antagonist (AT1 subtype) that inactivates bradykinin and does not suppress the enzyme kinase II. Reduces TPVR (total peripheral vascular resistance), the concentration of aldosterone and adrenaline in the blood, blood pressure (blood pressure), pressure in the vessels of the pulmonary circulation; reduces afterload and has a diuretic effect. Prevents the appearance of myocardial hypertrophy, improves tolerance to physical activity in patients with CHF (chronic heart failure).
Hydrochlorothiazide - a thiazide diuretic inhibits the reabsorption of sodium ions, increases the excretion of bicarbonate, potassium ions and phosphates in the urine. Reduces blood pressure by reducing BCC (circulating blood volume), suppressing the pressor effect of vasoconstrictor substances, changing the reactivity of the vascular wall and increasing the inhibitory effect on the ganglia.
Pharmacokinetics
- absorption: after oral administration, losartan and hydrochlorothiazide are rapidly absorbed from the gastrointestinal tract; bioavailability of losartan ~33%, time to reach Cmax (maximum concentration) of losartan is 1 hour, and its active metabolite is 3–4 hours;
- distribution: losartan binds to plasma proteins in 99%;
- metabolism: losartan undergoes presystemic elimination (the so-called first-pass effect through the liver), is metabolized in the liver by carboxylation, forms an active metabolite; hydrochlorothiazide is not metabolized by the liver;
- elimination: T ½ (half-life) of losartan is 1.5–2 hours, and its main metabolite is 3–4 hours; ~ 35% of the dose is excreted in urine, ~ 60% in feces; for hydrochlorothiazide T½ – 5.8–14.8 hours; ~ 61% is excreted unchanged in the urine.
When to use
To make everything clear, we must first look at the indications for use, and then the side effects of Lozap.
So, patients take the drug in the following cases:
- Hypertonic disease.
- Diabetic nephropathy.
- Chronic heart failure. In this case, the medicine is part of complex therapy.
The main reasons for its use are mentioned above, but there are also less obvious ones. These include left ventricular exhaustion. Doctors often prescribe medications to prevent hypertension. In this case, the side effects of Lozap pale in comparison to the benefits that the patient receives.
Rarely, but still the drug is prescribed in order to reduce the risk of complications associated with the functioning of blood vessels and the heart.
Contraindications
Absolute:
- hypokalemia/hypercalcemia, resistant to therapy;
- severe liver failure;
- refractory hyponatremia;
- obstruction of the biliary tract;
- anuria;
- hyperuricemia/gout;
- severe renal failure with CC (creatinine clearance) ≤ 30 ml/min;
- pregnancy period;
- lactation (breastfeeding);
- children and adolescents up to 18 years of age;
- increased individual sensitivity to sulfonamide derivatives, active and auxiliary ingredients of the drug.
Relative contraindications (Lozap Plus is taken with caution): bilateral renal artery stenosis or stenosis of the artery of a single kidney, hyponatremia (high risk of developing arterial hypotension in patients on a low- or salt-free diet), hypovolemic conditions (including vomiting, diarrhea ), hypomagnesemia, hypochloremic alkalosis, connective tissue diseases (including systemic lupus erythematosus), liver dysfunction or progressive liver disease, bronchial asthma (including a history), diabetes mellitus, aggravated allergic history, simultaneous taking NSAIDs (non-steroidal anti-inflammatory drugs) and cyclooxygenase (COX)-2 inhibitors, belonging to the Negroid race.
Instructions for use Lozap Plus: method and dosage
Lozap Plus tablets are taken orally, regardless of meal time.
Recommended dosage regimen according to indications:
- arterial hypertension: starting and maintenance dose – 1 tablet per day; if adequate blood pressure control is not achieved, the dose can be increased to the maximum – 2 tablets once a day; the maximum hypotensive effect of the drug is achieved within 3 weeks from the start of therapy;
- reducing the risk of developing cardiovascular pathologies and mortality in patients with arterial hypertension and left ventricular hypertrophy: starting dose of losartan – 50 mg/day; in the absence of achieving adequate control and target blood pressure levels during monotherapy with losartan, a combination of losartan with hydrochlorothiazide in a low dose (12.5 mg), which is provided by taking the drug Lozap Plus, is required; if necessary, the dose can be increased to 2 tablets 1 time per day (100 mg losartan + 25 mg hydrochlorothiazide).
Side effects
Frequency distribution scale of adverse reactions: very often ≥ 0.1; often ≥ 0.01 but < 0.1; uncommon ≥ 0.001 but < 0.01; rarely ≥ 0.0001 but <0.001; very rare < 0.0001, with unknown frequency - frequency cannot be calculated based on the available data.
In clinical studies of the losartan-hydrochlorothiazide combination, no additional side effects associated with their combined use were recorded. All side effects resulting from taking Lozap Plus were limited to those previously observed when using losartan or hydrochlorothiazide alone.
In controlled clinical trials of losartan and hydrochlorothiazide therapy for essential hypertension, the only adverse effect occurring at an incidence of ≥ 1% compared with placebo was dizziness. Other adverse reactions reported with the use of the losartan/hydrochlorothiazide combination in the treatment of essential hypertension:
- liver and biliary tract: rarely – hepatitis;
- laboratory and instrumental studies: rarely - increased activity of liver transaminases, hyperglycemia.
The use of losartan/hydrochlorothiazide may cause side effects specific to each component individually.
Losartan
- blood and lymphatic system: uncommon - anemia, ecchymosis, Henoch-Schönlein disease (hemorrhagic vasculitis), hemolysis;
- immune system: rarely - anaphylactic reactions, urticaria, angioedema (lips, face, pharynx, larynx, tongue);
- metabolism and nutrition: infrequently – gout, anorexia;
- psyche: often – insomnia; uncommon – anxiety, restlessness, panic attacks, depression, confusion, drowsiness, sleep disturbance, unusual dreams, memory impairment;
- nervous system: often – dizziness, headache; uncommon – paresthesia, increased excitability, peripheral neuropathy, migraine, tremor, fainting;
- organ of vision: uncommon – blurred vision, decreased visual acuity, burning sensation in the eye, conjunctivitis;
- organ of hearing and labyrinthine disorders: infrequently - tinnitus, vertigo;
- heart: infrequently - orthostatic hypotension, arterial hypotension, angina pectoris, pain in the sternum, atrioventricular (AV) block of the second degree, myocardial infarction, cerebrovascular disorders, arrhythmias (sinus bradycardia, atrial fibrillation, tachycardia, ventricular fibrillation, ventricular tachycardia), palpitations ;
- vessels: infrequently – vasculitis;
- respiratory system: often - upper respiratory tract infections, cough, nasal congestion, sinusitis; uncommon – laryngitis, pharyngitis, dyspnea, nosebleeds, bronchitis, rhinitis;
- Gastrointestinal tract: often - nausea, diarrhea, abdominal pain, dyspepsia; uncommon – vomiting, constipation, dry mouth, gastritis, flatulence, toothache;
- liver and bile ducts: with unknown frequency - impaired liver function;
- skin and subcutaneous tissues: uncommon - itching, rash, dermatitis, alopecia, dry skin, erythema, hyperemia, photosensitivity, hyperhidrosis;
- musculoskeletal system and connective tissue: often - muscle cramps, leg pain, back pain, sciatica; uncommon – muscle and bone pain, joint swelling, joint stiffness, arthritis, arthralgia, muscle weakness, fibromyalgia; with unknown frequency – rhabdomyolysis;
- kidneys and urinary tract: uncommon – urinary urgency, nocturia, urinary tract infections;
- reproductive system: uncommon – decreased libido/potency;
- the body as a whole: often – fatigue, asthenia, chest pain; uncommon – fever, swelling of the face;
- laboratory and instrumental studies: often - a slight decrease in hematocrit and hemoglobin, hyperglycemia; uncommon – slight increase in urea and creatinine levels; very rarely - increased levels of bilirubin and liver enzymes.
Hydrochlorothiazide
- hematopoietic system: uncommon – aplastic anemia, agranulocytosis, hemolytic anemia, purpura, leukopenia, thrombocytopenia;
- immune system: rarely - anaphylactic reactions up to anaphylactic shock;
- metabolism: uncommon – hyperglycemia, hyperuricemia, hypomagnesemia, hyponatremia, hypokalemia, hypochloremic alkalosis, hypercalcemia, anorexia;
- psyche: infrequently – insomnia;
- nervous system: infrequently – headache;
- organ of vision: infrequently – xanthopsia, temporary decrease in visual acuity;
- vessels: infrequently - cutaneous or necrotizing vasculitis;
- respiratory system: infrequently - RDS (respiratory distress syndrome), including non-cardiogenic pulmonary edema and pneumonitis;
- Gastrointestinal tract: uncommon – gastritis, nausea/vomiting, diarrhea, cramps, constipation, sialadenitis;
- liver and biliary tract: uncommon – cholecystitis, cholestatic jaundice, pancreatitis;
- skin and subcutaneous tissues: uncommon – urticaria, photosensitivity, toxic epidermal necrolysis;
- musculoskeletal system and connective tissue: infrequently – muscle cramps;
- kidneys and urinary tract: uncommon – interstitial nephritis, glycosuria, renal failure, impaired renal function;
- body as a whole: infrequently – dizziness, fever.
Overdose
There is no information on specific therapy for Lozap Plus overdose. It is recommended to stop taking the drug and monitor the patient's condition. Symptomatic therapy is indicated, including gastric lavage (if the tablets have been taken recently), elimination of electrolyte disturbances, dehydration and lowering blood pressure using standard methods - restoring blood volume and water-electrolyte balance.
Overdose typical for each of the components separately:
- losartan: the most frequently recorded symptoms of overdose are tachycardia and a pronounced decrease in blood pressure; bradycardia may be caused by stimulation of the parasympathetic nervous system. For symptomatic arterial hypotension, maintenance fluid therapy is recommended. Losartan, like its active metabolite, is not excreted by hemodialysis;
- Hydrochlorothiazide: The most commonly reported symptoms of overdose are the result of electrolyte deficiency (hypochloremia, hypokalemia, hyponatremia) and dehydration due to excessive diuresis. Concomitant use of cardiac glycosides during hypokalemia may aggravate the course of arrhythmias. There is no specific antidote, and the extent of hydrochlorothiazide elimination by hemodialysis is unknown.
Combination with alcohol
Taking medications together with alcoholic beverages, a person runs the risk of a sharp decrease in blood pressure, even to the point of fainting. Frivolous patients claim that the combination of Lozap Plus and alcohol is possible, if not every day, then every other day. But it should be borne in mind that in order to achieve a therapeutic effect, the medication should be taken continuously, without interruption.
Everyone knows that alcohol affects blood vessels, dilating them, and if there is already a substance in the blood that also acts, then there will be a rapid dilation of blood vessels, a decrease in their tone, and a significant drop in blood pressure. Too sharp a decrease in blood pressure is fraught with consequences:
- sudden weakness;
- dizziness;
- nausea;
- impaired coordination of movements;
- decreased temperature of the extremities.
Important! Combining antihypertensive drugs and alcohol is prohibited!
The most common causes of hypertension
special instructions
Losartan
Patients with a history of angioedema (swelling of the lips, face, pharynx and/or tongue) should be treated under close supervision.
In patients with hypovolemia and/or low sodium levels, symptomatic hypotension may develop due to dietary salt restriction, intensive use of diuretics, or diarrhea/vomiting (especially after the first dose). Correction of such conditions is required before starting therapy with Lozap Plus.
Water-electrolyte imbalances often occur in renal failure, so careful monitoring of plasma potassium levels and CK levels is necessary, especially in patients with heart failure with CK 30–50 ml/min. The combined use of Lozap Plus with potassium supplements, potassium-sparing diuretics and salt substitutes containing potassium is not recommended.
There is no experience with the use of the drug in patients who have recently undergone kidney transplantation.
In case of primary hyperaldosteronism, as a rule, there is no response to therapy with antihypertensive drugs that inhibit the renin-angiotensin system, therefore the use of Lozap Plus is not recommended.
An excessive decrease in blood pressure in patients with cerebrovascular disease or coronary artery disease, as with any other antihypertensive drugs, can cause the development of myocardial infarction or stroke.
In heart failure, with or without renal impairment, there is an increased risk of severe hypotension and renal impairment, often acute, as with other drugs that affect the renin-angiotensin system.
Particular caution is required when treating patients with obstructive hypertrophic cardiomyopathy or aortic/mitral stenosis, as with other vasodilators.
Losartan, similar to other ACE (angiotensin-converting enzyme) inhibitors, and angiotensin antagonists are less effective in lowering blood pressure in representatives of the Black race compared to representatives of other races. This may be due to more frequent episodes of low renin levels in representatives of this race in the population with arterial hypertension.
Hydrochlorothiazide
Hydrochlorothiazide, like any other antihypertensive drugs, may potentiate symptomatic hypotension in some patients. Therefore, it is necessary to monitor the appearance of such clinical signs of water-electrolyte imbalance as hyponatremia, hypovolemia, hypochloremic alkalosis, hypokalemia or hypomagnesemia, which can develop against the background of concomitant vomiting or diarrhea. In such patients, serum electrolyte levels should be checked periodically (after a certain time). In the presence of swelling in hot weather, hypervolemic hyponatremia may develop.
Therapy with thiazide diuretics can lead to impaired glucose tolerance, which may require dose adjustment of antidiabetic agents, including insulin. Taking thiazides by patients with impaired glucose tolerance is fraught with the manifestation of diabetes mellitus.
Thiazide diuretics can inhibit urinary calcium excretion and potentiate intermittent minor increases in serum calcium levels. Severe hypercalcemia may be a sign of latent hyperparathyroidism. For upcoming parathyroid function tests, hydrochlorothiazide should be discontinued before diagnosis.
The use of thiazide diuretics may result in an increase in blood levels of triglycerides and cholesterol.
Due to the use of thiazide diuretics, some patients may develop hyperuricemia and/or gout. Because losartan reduces uric acid levels, its use in combination with hydrochlorothiazide may slow the development of diuretic-induced hyperuricemia.
During therapy with thiazides, patients with a history of hypersensitivity and/or bronchial asthma may develop allergic reactions; Cases of the onset or exacerbation of SLE (systemic lupus erythematosus) have been described. Allergic reactions can also be caused by the Crimson dye [Ponceau 4R] contained in the tablets.
Impact on the ability to drive vehicles and complex mechanisms
The effect of Lozap Plus on the ability to perform types of work requiring a high speed of psychomotor reactions and increased concentration has not been studied. But, given the side effects of therapy with antihypertensive drugs, such as dizziness or drowsiness, driving vehicles or operating complex machinery should be done with caution, especially when starting treatment or increasing the dose of the drug.
Use during pregnancy and lactation
Effect of active components of Lozap Plus on pregnancy:
- hydrochlorothiazide: experience with its use, especially in the first trimester, is limited and there is insufficient data from animal studies. Hydrochlorothiazide is known to cross the placental barrier and is found in the umbilical cord blood. Considering the pharmacological mechanism of action of the substance, it can be assumed that when used by pregnant women, fetoplacental blood flow may worsen and cause pathologies in the fetus/newborn such as jaundice, thrombocytopenia and electrolyte imbalance;
- losartan: it is known that angiotensin II receptor antagonists, when used in the second and third trimesters of pregnancy, have a fetotoxic effect, expressed by decreased renal function, oligohydramnios, delayed ossification of the skull, and also exhibit toxicity to the newborn, causing renal failure, arterial hypotension and hyperkalemia.
When using Lozap Plus by pregnant women in the second and third trimesters, it is recommended to perform an ultrasound (ultrasound examination) of the skull and kidneys of the fetus.
Newborns whose mothers took Lozap Plus during pregnancy need careful monitoring for the development of arterial hypotension.
When planning pregnancy, it is necessary to switch to alternative antihypertensive treatment options with an established safety profile. If pregnancy is diagnosed during therapy with Lozap Plus, the drug should be immediately interrupted and alternative treatment should be chosen.
Lozap Plus tablets are contraindicated during pregnancy!
There is no data on the use of Lozap Plus during lactation. It is known that hydrochlorothiazide passes into breast milk, and thiazide diuretics can cause intense diuresis and can inhibit milk production. Therefore, the use of the drug is contraindicated during breastfeeding.
Why can't it be used during breastfeeding or pregnancy?
Above we described the side effects of Lozap with long-term use. But judging by the reviews, many people believe that the drug, which should not be taken by pregnant women, is harmful to everyone. Is it so? Let's figure it out now.
Why does the instruction not allow the use of the drug in pregnant women? This happens because there is no data on the use of the drug. However, it is known that a medication that affects the renin-angiotensin system when used in the second and third trimester can cause fetal death or developmental defects. This is the reason for the use of the drug during pregnancy.
If there is a need to use the drug while a woman is breastfeeding, then the question of what is more important is to decide - stopping treatment or stopping feeding.
For impaired renal function
Losartan, like other substances that affect the RAAS, may increase serum levels of urea and creatinine in patients with bilateral renal artery stenosis or with renal artery stenosis of a solitary kidney. There is evidence of impaired renal function as a result of inhibition of the RAAS, for example, in pre-existing renal failure or in severe heart failure. Changes in renal function may be reversible and decrease after discontinuation of the drug.
According to the instructions, Lozap Plus is recommended to be taken with caution in case of bilateral renal artery stenosis or renal artery stenosis of a single kidney.
The use of Lozap Plus in cases of severe renal dysfunction with CC ≤ 30 ml/min is contraindicated.
What do doctors think?
Reviews from doctors also differ. Some consider the drug to be effective in cases where hypertension is present in a mild form. Other doctors believe that using the drug for severe hypertension in combination with other heart diseases is pointless. In this case, the effectiveness of the drug is quite low. For this reason, patients are forced to additionally use stronger medications.
To summarize, in general, doctors believe that the drug is good, but it should be used only at certain stages of the disease, otherwise it is pointless.
Doctors also remind that unauthorized use of medication can only worsen the course of the disease. For this reason, you should always consult your doctor.
For liver dysfunction
According to pharmacokinetic studies, a marked increase in plasma concentrations of losartan is observed in patients with liver cirrhosis. Thiazide diuretics, including hydrochlorothiazide, can cause intrahepatic cholestasis, and minor disturbances in water and electrolyte balance can provoke the development of hepatic coma. In this connection, Lozap Plus is prescribed with caution in cases of liver dysfunction (including a history) or with progressive liver diseases. In case of severe liver dysfunction, the use of the drug is contraindicated.
Drug interactions
Losartan
- rifampicin, fluconazole: cases of decreased concentrations of the active metabolite of losartan have been described without assessing clinical data;
- Potassium-sparing diuretics (spironolactone, triamterene, amiloride), potassium supplements or salt substitutes containing potassium: may increase serum potassium levels; the combined use of these drugs is not recommended;
- lithium salts: losartan may slow down the excretion of lithium, so careful monitoring of their levels in the blood serum is necessary;
- nonsteroidal anti-inflammatory drugs (NSAIDs), for example, selective COX-2 inhibitors; acetylsalicylic acid in doses used for anti-inflammatory action; non-selective NSAIDs: may weaken the antihypertensive effect of Lozap Plus; the risk of deterioration of kidney function increases, including acute renal failure; an increase in the level of potassium in the blood serum is possible, especially with initial impairment of renal function. Combined use is prescribed with caution, especially in the elderly, with mandatory provision of patients with adequate hydration and monitoring of renal function at the beginning of joint use and periodically during therapy; renal dysfunction is usually reversible;
- antipsychotic drugs, amifostine, baclofen, tricyclic antidepressants, other drugs that cause hypotension: the risk of arterial hypotension increases.
Hydrochlorothiazide
- barbiturates, alcohol, opioid analgesics, antidepressants: the risk of orthostatic hypotension increases;
- antidiabetic drugs (insulin and oral hypoglycemic agents): hydrochlorothiazide can affect their glucose tolerance, which may require dose adjustment;
- metformin: possible development of lactic acidosis as a consequence of functional renal failure caused by the use of hydrochlorothiazide; Caution should be exercised when used together;
- other antihypertensive drugs: synergism of action contributes to the development of an additive effect;
- cholestyramine, colestipol: ion exchange resins inhibit the absorption of hydrochlorothiazide; a single dose of cholestyramine/colestipol leads to the binding of hydrochlorothiazide and reduces its absorption from the gastrointestinal tract by 85%/43%;
- corticosteroids, adrenocorticotropic hormone (ACTH): may aggravate electrolyte deficiency, especially hypokalemia;
- pressor amines (adrenaline): a decrease in effect is possible, but does not preclude their use;
- non-depolarizing muscle relaxants (tubocurarine chloride): hydrochlorothiazide may enhance their effect;
- lithium preparations: diuretics, including hydrochlorothiazide, reduce their renal clearance, significantly increasing the risk of toxic effects of lithium; simultaneous use is recommended to be avoided;
- anti-gout drugs (sulfinpyrazone, probenecid, allopurinol): dosage adjustment may be required because hydrochlorothiazide can increase serum uric acid levels; there is likely to be an increase in allergic reactions to allopurinol;
- anticholinergic drugs (atropine, biperidine): can increase the bioavailability of hydrochlorothiazide by inhibiting gastrointestinal motility and reducing the rate of gastric emptying;
- cytotoxins (methotrexate, cyclophosphamide): it is possible to inhibit their excretion through the kidneys and enhance the myelosuppressive effect;
- salicylates: when used in high doses, their toxic effect on the central nervous system (CNS) may increase;
- methyldopa: isolated episodes of hemolytic anemia have been described;
- cyclosporine: increases the risk of hyperuricemia and complications of gout;
- cardiac glycosides: hydrochlorothiazide-induced hypokalemia/hypomagnesemia may contribute to the development of arrhythmias induced by digitalis preparations;
- digitalis glycosides, antiarrhythmic drugs (the therapeutic effect of which depends on serum potassium levels), class IA antiarrhythmic drugs (hydroquinidine, quinidine, disopyramide), class III antiarrhythmic drugs (amiodarone, dofetilide, ibutilide, sotalol), some antipsychotics (thioridazine, levomepromazine, chlorpromazine , trifluoperazine, cyamemazine, sultopride, sulpiride, amisulpride, pimozide, tiapride, droperidol, haloperidol), other drugs that can cause torsade de pointes (difemanil, bepridil, cisapride, erythromycin intravenously, halofantrine, pentamidine, mizolastine, terfenadine, vincamycin intravenously but) : It is recommended to regularly monitor serum potassium levels, since hypokalemia is a factor predisposing to the development of torsade de pointes; electrocardiogram (ECG) monitoring is also necessary;
- calcium salts: hydrochlorothiazide may increase serum calcium levels by reducing its excretion; monitoring of serum calcium levels and appropriate adjustment of the dosage regimen of calcium preparations is required; due to its effect on calcium metabolism, hydrochlorothiazide may distort the results of tests assessing the function of the parathyroid glands;
- Carbamazepine: Clinical observation and laboratory monitoring of blood sodium levels is required in patients using carbamazepine due to the risk of developing symptomatic hyponatremia;
- iodine-containing contrast agents: with dehydration caused by diuretics, the risk of developing acute renal failure increases, especially when taking iodine preparations in high doses, so rehydration is required before their administration;
- amphotericin B (parenteral), corticosteroids, ACTH, stimulant laxatives or glycyrrhizin (found in licorice): hydrochlorothiazide can cause electrolyte deficiency, especially hypokalemia.
Reviews about Lozap Plus
Patients who have chosen Lozap Plus to lower their blood pressure leave mostly positive reviews about it. They write that taking tablets once a day significantly improves well-being, steadily lowers blood pressure, and stops dizziness. Some are concerned about the diuretic effect of the drug and wonder whether it is harmful with prolonged use.
Patients with a tendency to swelling are forced to refuse therapy with Lozap Plus, since diuretics are contraindicated when taking it, or alternate its use with courses of other antihypertensive drugs.
Pharmacodynamics of the drug
One of the active ingredients of Lozap Plus is losartan. It is contained in the drug at a concentration of 50 mg per tablet.
This drug is a synthetic angiotensin-2 blocker. It binds to AT-1 receptors, blocking them. Thanks to this, anigotensin-2 cannot have effects on the body that lead to hypertension. The drug inhibits absolutely the entire enzyme angiotensin-2, regardless of its origin. Therefore, it has a powerful hypotensive effect.
This drug also blocks the production of aldosterone. Due to this, large amounts of potassium are not excreted.
Lozap Plus does not have a suppressive effect on other hormones. It does not affect ACE and does not affect the breakdown of bradykinin. Therefore, when taking it, such a side effect as a dry cough rarely occurs.
When angiotensin-2 is inhibited, its negative feedback is eliminated, which leads to an increase in the concentration of renin in the body. The amount of free angiotensin-2, which is inactive because it cannot contact receptors blocked by the drug, will also increase.
Losartan helps reduce proteinuria, as well as plasma uric acid concentrations. It also reduces the heart rate, reduces the load on the heart, increases the cardiac index and prevents the pulmonary capillaries from jamming under pressure.
This active ingredient Lozapa also reduces the concentration of norepinephrine in the blood. However, this effect does not last long.
Hydrochlorothiazide is a thiazide diuretic. It is a substance that has a diuretic effect.
This active ingredient helps remove more sodium and chlorine from the body. Thanks to this, excess fluid comes out and swelling of all tissues, including blood vessels, is eliminated, which leads to an increase in the gaps in them. The volume of blood circulating throughout the body also decreases, which also leads to a decrease in the load on the heart.
This substance promotes the active production of aldosterone, which provokes the excretion of potassium in large quantities. However, this combination of hydrochlorothiazide and losartan blocks this hormone, reducing the likelihood of hypokalemia.
Also, the combination of these substances enhances their hypotensive properties. At the same time, the effectiveness of the diuretic is not affected by the gender, age and race of the person. Therefore, dosage adjustment is not required in these cases.
The effect of the drug is observed approximately two hours after taking it. It continues throughout the day.