Release form and composition
Dosage forms of release of Trimetazidine MV:
- extended-release film-coated tablets: light pink to pink, round, biconvex; the core in cross section is white or almost white (10, 20 or 30 pcs. in blister packs, 1–6 or 10 packs in a cardboard pack; 10, 20, 30, 40, 50, 60 or 100 pcs. in polymer cans, 1 can in a cardboard pack);
- modified-release, film/coated tablets: pink to brownish-pink, round, biconvex, scored on one side; kernel on a cross section - from almost white or white to white with a yellowish tint (15 pcs. in blister packs, 2 or 4 packs in a cardboard pack; 10 pcs. in blister packs, 1–3 in a cardboard pack , 6, 12 or 18 packs; 20 pcs each in blister packs, 1–3 packs in a cardboard pack).
Composition of 1 extended-release tablet:
- active substance: trimetazidine dihydrochloride – 35 mg;
- additional components: magnesium stearate – 1.55 mg; colloidal silicon dioxide – 1.55 mg; hypromellose – 93 mg; microcrystalline cellulose – 178.9 mg;
- shell: Opadry II 85F240012 pink (macrogol-3350 – 2.438 mg; talc – 1.48 mg; polyvinyl alcohol – 4 mg; red iron oxide dye – 0.04 mg; yellow iron oxide dye – 0.022 mg; titanium dioxide – 2, 02 mg) – 10 mg.
Composition of 1 modified-release tablet:
- active substance: trimetazidine dihydrochloride – 35 mg;
- additional components: colloidal silicon dioxide – 2 mg; anhydrous calcium hydrogen phosphate – 157 mg; hypromellose – 50 mg; Magnesium stearate – 1.5 mg; crospovidone – 2.5 mg; talc – 2 mg;
- shell: Opadry II pink (85F34610) (polyvinyl alcohol - 40%; titanium dioxide - 24.24%; macrogol 3350 - 20.2%; talc - 14.8%; yellow iron oxide dye - 0.37%; oxide dye red iron – 0.36%; black iron oxide dye – 0.03%) – 8 mg.
Pharmacological properties
Pharmacodynamics
Trimetazidine MB has an antihypoxic effect.
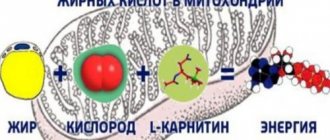
Directly affects brain neurons and cardiomyocytes, optimizing their function and metabolism. The cytoprotective effect is due to the activation of oxidative decarboxylation, an increase in energy potential and a rationalization of oxygen consumption (due to the inhibition of long-chain 3-ketoacyl-CoA thiolase, an increase in aerobic glycolysis and blockade of fatty acid oxidation are observed).
Main properties of trimetazidine:
- maintaining myocardial contractility, preventing intracellular depletion of creatine phosphate and adenosine triphosphate;
- normalization of the functioning of membrane ion channels in conditions of acidosis, preventing the accumulation of sodium and calcium ions in cardiomyocytes, normalization of the intracellular concentration of potassium ions;
- reduction of intracellular acidosis and increased phosphate content, which is caused by reperfusion and myocardial ischemia;
- preventing the damaging effects of free radicals, maintaining the integrity of cell membranes, preventing the activation of neutrophils in the ischemic zone, increasing the duration of the electrical potential, reducing the release of creatine phosphokinase from cells and the severity of ischemic damage to the myocardium.
The substance does not have a direct effect on hemodynamic parameters.
The main effects of Trimetazidine MV for angina pectoris:
- limiting exercise-induced blood pressure fluctuations without significant changes in heart rate;
- an increase in coronary reserve, which leads to a slowdown in the onset of ischemia associated with physical activity (starting from the 15th day of treatment);
- improvement of contractile function of the left ventricle against the background of ischemic dysfunction;
- reducing the incidence of angina attacks and the need for short-acting nitroglycerin.
Pharmacokinetics
Trimetazidine after oral administration is almost completely and quickly absorbed from the gastrointestinal tract. Its bioavailability is 90%. The time to reach maximum plasma concentration in the blood is approximately 5 hours. Over 24 hours, the concentration of the substance in the blood plasma is maintained at a level greater than and equal to 75% of the maximum concentration determined over 11 hours. With regular use, equilibrium concentration is achieved after 60 hours. Concomitant food intake does not affect the bioavailability of trimetazidine.
The volume of distribution of the substance is 4.8 l/kg. Trimetazidine is characterized by a fairly low degree of binding to plasma proteins - approximately 16%.
The substance is excreted from the body mainly by the kidneys (approximately 60% unchanged). In healthy young volunteers, the half-life is 7 hours; in patients over 65 years of age, it is approximately 12 hours.
The clearance of trimetazidine is characterized to a greater extent by renal clearance, which directly correlates with creatinine clearance, and to a lesser extent by hepatic clearance, which decreases with patient age.
In patients over 75 years of age, the plasma concentration of trimetazidine in the blood may increase, which is associated with an age-related decrease in renal function. No safety differences were found in this group of patients compared with the general population.
In patients with renal failure, the plasma concentration of trimetazidine in the blood is increased on average by 2.4/4 times (in patients with creatinine clearance 30-60 or <30 ml/min, respectively) compared to volunteers without impaired renal function. No safety differences were found in this group of patients compared with the general population.
Trimetazidine MR
Suction:
After oral administration, trimetazidine is quickly and almost completely absorbed from the gastrointestinal tract. Bioavailability - 90%. The time to reach the maximum concentration in the blood plasma is about 5 hours. Over 24 hours, the concentration of trimetazidine in the blood plasma is maintained at a level greater than and equal to 75% of the maximum concentration determined within 11 hours. The equilibrium concentration with regular use is achieved after 60 hours. Simultaneous use food does not affect the bioavailability of trimetazidine.
Distribution:
The volume of distribution is 4.8 l/kg. The degree of binding to plasma proteins is quite low - about 16% in vitro.
Removal:
Trimetazidine is excreted from the body primarily by the kidneys (about 60% unchanged). The half-life in young healthy volunteers is about 7 hours, in patients over 65 years of age - about 12 hours.
The clearance of trimetazidine is characterized to a greater extent by renal clearance, which directly correlates with creatinine clearance (CC), and to a lesser extent by hepatic clearance, which decreases with patient age.
Special groups
Patients over 75 years of age
In patients over 75 years of age, increased plasma concentrations of trimetazidine may occur due to age-related decline in renal function. A special study was conducted in a population of patients over 75 years of age while taking trimetazidine tablets 35 mg 2 times a day. An analysis performed by the kinetic population method showed an average twofold increase in plasma concentrations in patients with severe renal failure (creatinine clearance less than 30 ml/min) compared with patients with creatinine clearance more than 60 ml/min.
No safety differences were found in patients over 75 years of age compared with the general population.
Patients with kidney failure
The concentration of trimetazidine in blood plasma was increased on average by 2.4 times in patients with moderate renal failure (creatinine clearance 30-60 ml/min) and on average by 4 times in patients with severe renal failure (creatinine clearance less than 30 ml/min) compared to healthy volunteers with normal renal function.
No significant safety differences were observed in this patient population compared with the general population.
Use in children and adolescents
The pharmacokinetics of trimetazidine in children and adolescents under 18 years of age have not been studied.
Contraindications
Absolute:
- severe renal failure (in patients with creatinine clearance <30 ml/min);
- tremor, parkinsonism symptoms, Parkinson's disease, restless legs syndrome;
- age under 18 years;
- pregnancy and breastfeeding;
- individual intolerance to any component of the drug.
Relative (Trimetazidine MB should be prescribed under medical supervision):
- moderate renal failure (in patients with creatinine clearance 30–60 ml/min);
- age from 75 years.
Trimetazidine MV-Teva
Pharmacodynamics Has an antihypoxic effect. Trimetazidine prevents a decrease in intracellular adenosine triphosphate (ATP) concentration by maintaining the energy metabolism of cells in a state of hypoxia. Thus, the drug ensures the normal functioning of membrane ion channels, transmembrane transport of potassium and sodium ions and the preservation of cellular homeostasis. Trimetazidine inhibits the oxidation of fatty acids due to the selective inhibition of the enzyme 3-ketoacyl-CoA thiolase (3-CAT) of the mitochondrial long-chain isoform of fatty acids, which leads to increased oxidation of glucose and acceleration of glycolysis with oxidation of glucose, which determines the protection of the myocardium from ischemia. The switch of energy metabolism from fatty acid oxidation to glucose oxidation underlies the pharmacological properties of trimetazidine.
It has been experimentally confirmed that trimetazidine has the following properties:
supports energy metabolism of the heart and neurosensory tissues during ischemia; reduces the severity of intracellular acidosis and changes in transmembrane ion flow that occur during ischemia; reduces the level of migration and infiltration of polynuclear neutrophils in ischemic and reperfused heart tissues; reduces the size of myocardial damage; does not have a direct effect on hemodynamic parameters. In patients with angina, trimetazidine:
increases coronary reserve, thereby slowing down the onset of ischemia caused by physical activity, starting from the 15th day of therapy; limits exercise-induced blood pressure fluctuations without significant changes in heart rate; reduces the frequency of angina attacks and the need for short-acting nitroglycerin; improves contractile function of the left ventricle in patients with ischemic dysfunction. Pharmacokinetics After oral administration, trimetazidine is absorbed from the gastrointestinal tract and reaches maximum plasma concentrations after approximately 5 hours. Over 24 hours, the concentration in the blood plasma remains at a level exceeding 75% of the concentration determined after 11 hours. The equilibrium state is reached after 60 hours. Food intake does not affect the bioavailability of trimetazidine.
The volume of distribution is 4.8 l/kg, which indicates good distribution of trimetazidine in tissues (the degree of binding to plasma proteins is quite low, about 16% in vitro). Trimetazidine is excreted mainly by the kidneys, mainly unchanged. Renal clearance of trimetazidine directly correlates with creatinine clearance (CC), hepatic clearance decreases with patient age.
Instructions for use of Trimetazidine MB: method and dosage
Trimetazidine MB is taken orally, without chewing, whole, with water, preferably with meals.
Recommended dosage regimen: 2 times a day (morning and evening), 1 tablet (maximum).
The duration of the course is determined by the doctor individually.
For patients with moderate renal failure, including elderly patients (with a creatinine clearance of 30–60 ml/min), Trimetazidine MB 35 mg tablets should be taken once a day in the morning. It is necessary to select the dose for patients over 75 years of age with extreme caution.
Trimetazidine mv-teva tablet p/o film prolong. 35mg 60 pcs
Pharmacological group:
antihypoxic drug
Pharmacodynamics:
Trimetazidine under conditions of ischemia and hypoxia prevents the intracellular decrease in adenosine triphosphatase (ATP) activity, maintaining energy metabolism and ensuring cell homeostasis by ensuring the normal functioning of ion channels of cell membranes and transmembrane sodium-potassium flow. It inhibits beta-oxidation of fatty acids by selectively blocking the enzyme 3-ketoacylCoA thiolase, which enhances glucose oxidation. Cells in a state of ischemia require less oxygen to obtain energy from glucose oxidation than from beta-oxidation of fatty acids.
The switching of cellular energy metabolism from fatty acid oxidation to glucose oxidation underlies the pharmacological effects of trimetazidine. Under experimental conditions, it has been shown that the drug: - supports the energy metabolism of the heart and neurosensory tissues under ischemic conditions; — reduces the severity of intracellular acidosis and changes in the transmembrane ion flow that occur during ischemia; - reduces the migration and infiltration of polynuclear neutrophils in ischemic and reperfused cardiac tissues; - reduces the size of myocardial damage; - does not have an adverse effect on hemodynamic parameters.
In patients with coronary heart disease, trimetazidine acts as a metabolic agent, maintaining sufficient intracellular activity of high-energy phosphates in the myocardium. The anti-ischemic effect is achieved without affecting hemodynamics.
In patients with angina pectoris, trimetazidine: - increases coronary reserve, thereby slowing down the onset of exercise-induced ischemia, starting from the 15th day of therapy; - limits fluctuations in blood pressure caused by physical activity, without significant changes in heart rate; - reduces the frequency of angina attacks and the need for short-acting nitroglycerin; — improves the contractile function of the left ventricle in patients with ischemic dysfunction.
Pharmacokinetics:
When taken orally, trimetazidine is rapidly absorbed from the gastrointestinal tract (GIT), its maximum concentration in blood plasma is reached on average within 5 hours.
For more than 24 hours, trimetazidine plasma concentrations are maintained at levels greater than or equal to 75% of the maximum.
The equilibrium state of drug concentration in the blood is achieved after 60 hours.
Food intake does not affect the pharmacokinetic properties of trimetazidine. The volume of distribution is 4.8 l/kg. The binding to plasma proteins is low and in vitro is 16%.
Trimetazidine is excreted mainly by the kidneys, mainly unchanged.
When taken orally at a dose of 35 mg, the half-life in young healthy volunteers averages 7 hours, in patients over 65 years of age - 12 hours.
Total renal clearance of trimetazidine directly correlates with creatinine clearance (CC), and hepatic clearance decreases with age.
Patients over 75 years of age: Patients over 75 years of age may experience increased plasma exposure of trimetazidine as a result of age-related declines in renal function. No differences were found regarding the safety of the drug in patients over 75 years of age compared to the general population.
Patients with impaired renal function Plasma exposure of trimetazidine was increased approximately 2.4-fold in patients with moderate renal impairment (creatinine clearance 30-60 ml/min), and approximately 4-fold in patients with severe renal impairment degrees (creatinine clearance less than 30 ml/min) compared with healthy volunteers with normal renal function.
No differences were found regarding the safety of the drug in patients with impaired renal function compared to the general population.
Use in children and adolescents The pharmacokinetics of trimetazidine in children and adolescents under 18 years of age have not been studied.
Side effects
Possible adverse reactions (> 10% - very common; > 1% and < 10% - often; > 0.1% and < 1% - uncommon; > 0.01% and < 0.1% - rare; < 0. 01%, including individual messages - very rarely; with an unspecified frequency - in cases where it is not possible to estimate the frequency of occurrence of the violation):
- nervous system: often – dizziness, headache; with an unknown frequency - symptoms of parkinsonism (manifested in the form of tremor, akinesia, increased tone), unsteadiness of gait and instability in the Romberg position, restless legs syndrome and other disorders of the musculoskeletal system (usually reversible), sleep disorders (manifested in the form of insomnia , drowsiness);
- digestive system: often - nausea, abdominal pain, dyspepsia, diarrhea, vomiting; with unknown frequency – hepatitis, constipation;
- cardiovascular system: rarely - a pronounced decrease in blood pressure, tachycardia, palpitations, flushing of the face, extrasystole, orthostatic hypotension, which can occur with general weakness, loss of balance or dizziness, especially when combined with drugs with antihypertensive effects ;
- hematopoietic organs: with unknown frequency - thrombocytopenia, agranulocytosis, thrombocytopenic purpura;
- skin: often – itching, skin rash, urticaria; with an unknown frequency - acute generalized exanthematous pustulosis;
- allergic reactions: with an unknown frequency - Quincke's edema;
- other: often – asthenia.
special instructions
Trimetazidine MB is not intended for the relief of angina attacks. Also, the drug should not be used for the initial course of treatment of unstable angina or myocardial infarction before/during the first days of hospitalization. In cases of an attack of angina, it is necessary to review and adapt therapy (use of medications or a revascularization procedure).
While taking Trimetazidine MB, the appearance/worsening of symptoms of parkinsonism (akinesia, tremor, increased tone) is possible, and therefore regular monitoring of patients, especially the elderly, is indicated. In doubtful cases, patients should be referred to a neurologist for appropriate examination.
If symptoms of parkinsonism, restless legs syndrome, tremor, instability in the Romberg position, unsteadiness of gait and other movement disorders appear, Trimetazidine MB should be permanently discontinued. Such cases are rare; symptoms usually disappear after cessation of therapy (most often within 4 months after discontinuation of Trimetazidine MB; if symptoms persist longer than this period, you should consult a neurologist).