- July 30, 2020
- Infectious
- Olga Simchenko
Amoxiclav 125 mg suspension is an antibiotic intended for systemic use. The medicine is available in the form of a white or yellowish powder. The drug is prescribed for the treatment of diseases caused by bacterial infections. The medicine can be taken by children from an early age, but it must be remembered that it has certain indications and contraindications that are important to consider.
Compound
Each 5 ml of suspension 125 mg + 31.25 mg contains:
- active substances: amoxicillin (in the form of trihydrate) in terms of the active substance - 125 mg; clavulanic acid (in the form of potassium salt) in terms of the active substance - 31.25 mg;
- excipients: citric acid (anhydrous) - 2.167 mg; sodium citrate (anhydrous) - 8.335 mg; sodium benzoate - 2.085 mg; microcrystalline cellulose and carmellose sodium - 28.1 mg; xanthan gum - 10.0 mg; colloidal silicon dioxide - 16.667 mg; silicon dioxide - 0.217 g; strawberry flavoring - 15,000 mg; sodium saccharinate - 5,500 mg; mannitol up to 1250 mg.
Price
Dear readers, the price list with prices for Amoxiclav 500 mg + 125 mg tablets is updated on our website once a day, which is why the prices indicated in it may differ from the real ones. Hope for your understanding.
| Drug name | Price for 1 unit, rub. | Price per package, rub. | Link |
| Amoxiclav® film-coated tablets 500 mg + 125 mg, 15 pcs. | |||
| 23.87 | 358.00 | Buy | |
| 23.8 | 357.00 | Buy | |
| Amoxiclav® film-coated tablets 500 mg + 125 mg, 14 pcs. | |||
| 23.29 | 326.00 | Buy | |
| Amoxiclav® film-coated tablets 500 mg + 125 mg, 15 pcs. | |||
| 27.36 | 410.47 | Buy |
Mechanism of action
Amoxicillin is a semisynthetic broad-spectrum antibiotic that is active against many gram-positive and gram-negative microorganisms. At the same time, amoxicillin is susceptible to destruction by beta-lactamases, and therefore the spectrum of activity of amoxicillin does not extend to microorganisms that produce this enzyme.
Clavulanic acid is a beta-lactamase inhibitor, structurally related to penicillins, and has the ability to inactivate a wide range of beta-lactamases found in microorganisms resistant to penicillins and cephalosporins. Clavulanic acid is sufficiently effective against plasmid beta-lactamases, which most often cause bacterial resistance, and is not effective against type I chromosomal beta-lactamases, which are not inhibited by clavulanic acid.
The presence of clavulanic acid in the drug protects amoxicillin from destruction by enzymes - beta-lactamases, which expands the antibacterial spectrum of amoxicillin.
Amoxiclav has antibacterial activity against the following microorganisms:
- Gram-positive anaerobes (Staphylococcus aureus, pneumococcus, streptococcus pyogenes, other species of the genus Staphylococcus and Streptococcus, clostridia, peptococci);
- Gram-negative anaerobes (Coli bacterium, bacteria of the genus Enterobacter, Klebsiella, Moraxella catharalis, Bordetella, Salmonella, Shigella, Vibrio cholerae).
Due to the fact that some strains of the above bacteria produce beta-lactamase, this makes them insensitive to Amoxicillin monotherapy.
Reviews
Parents' opinion
Screenshot from one of the most popular review sites. 81% of users recommend the antibiotic Amoxiclav
On the Internet you can find many positive reviews about Amoxiclav suspension. Parents note that it has a pleasant taste and aroma, so most children drink it with pleasure. They also like that the kit comes with a syringe, which is convenient for dosing the drug.
Negative reviews are due to the fact that the antibiotic can cause allergies and other side effects.
Doctor Komarovsky's opinion
Evgeniy Olegovich believes that antibiotics should only be prescribed by a doctor, taking into account the pathogen, diagnosis, their compatibility with another drug and a number of other factors. They can be taken according to strict indications and only for bacterial infections, and in viral diseases they can cause complications. And you shouldn’t take antibiotics for prophylaxis.
The antibiotic can be bought strictly according to a prescription, so self-medication is unacceptable; only the doctor should decide how to give Amoxiclav to the child.
By
Pharmacokinetics
Both active substances are well absorbed, regardless of food intake. The maximum concentration in plasma is reached one hour after taking the drug (Cmax for amoxicillin - 3-12 μg/ml, Cmax for clavulanic acid - 2 μg/ml.
The components of Amoxiclav are well distributed in pleural, parietal, synovial fluids, bronchial secretions, secretions of the nasal sinuses, saliva, as well as in body tissues (lungs, tonsils, middle ear, ovaries, uterus, liver, prostate gland, muscle tissue, gall bladder ). The drug is not able to penetrate the blood-brain barrier (with non-inflamed meninges). Penetrates the placental barrier and is excreted in trace concentrations in breast milk. It binds poorly to plasma proteins, amoxicillin trihydrate disintegrates partially, clavulanic acid completely.
The drug is excreted by the kidneys almost unchanged. Small amounts are excreted by the lungs and through the intestines. The half-life is 1-1.5 hours.
Amoxicillin is excreted by the kidneys almost unchanged by tubular secretion and glomerular filtration. Small amounts may be excreted through the intestines and lungs. T1/2 of amoxicillin and clavulanic acid is 1-1.5 hours. In case of severe renal failure, it increases for amoxicillin to 7.5 hours, for clavulanic acid - up to 4.5 hours. Both components are removed during hemodialysis.
Amoxiclav 2X tablets p/o 875 mg 125 mg package No. 14
Amoxiclav 2x Trade name of the drug: Amoxiclav 2x, film-coated tablets 875 mg/125 mg. International nonproprietary name: Amoxicillin, clavulanic acid/Amoxicikllin, clavulanic acid.
Description White or almost white, oval, biconvex tablets with beveled edges, film-coated, scored on one side and extruded 875/125, AMC is extruded on the other side.
Composition Active ingredients: amoxicillin and clavulanic acid. Each tablet contains 875 mg amoxicillin trihydrate and 125 mg clavulanic acid potassium salt (7:1 ratio). Excipients Core: colloidal anhydrous silicon dioxide, crospovidone, croscarmellose sodium, magnesium stearate, microcrystalline cellulose; shell: hydroxypropyl cellulose, ethyl cellulose, polysorbate 80, triethyl citrate, talc, titanium dioxide E 171.
Pharmacotherapeutic group Antibacterial agents for systemic use; combinations of penicillins, including beta-lactamase inhibitors. ATX code: DO1SK.02.
Pharmacological properties Pharmacodynamics Amoxicillin is a semi-synthetic penicillin (beta-lactam antibiotic) that inhibits one or more enzymes (often called penicillin-binding proteins) in the biosynthesis of peptidoglycan, an integral component of the bacterial cell wall. Inhibition of peptidoglycan synthesis leads to loss of cell wall strength, which usually causes cell lysis and death. Amoxicillin is destroyed by beta-lactamases produced by resistant bacteria, so it is inactive against microorganisms that produce these enzymes. Clavulanic acid is a beta-lactam structurally similar to penicillins. It inhibits some beta-lactamases and thereby prevents the inactivation of amoxicillin. Clavulanic acid alone does not have a clinically useful antibacterial effect. The time to maintain concentrations above the minimum inhibitory concentration (T > MIC) is recognized as the main determinant of the effectiveness of amoxicillin. Mechanisms of resistance There are two main mechanisms of bacterial resistance to amoxicillin/clavulanic acid: - inactivation by bacterial beta-lactamases that are insensitive to the inhibitory effect of clavulanic acid, including beta-lactamases of classes B, C and D; - a change in penicillin-binding proteins, as a result of which the affinity of antibacterial drugs for target structures decreases. Impermeability of bacteria or mechanisms of active drug transport from the bacterial cell can directly cause or contribute to resistance, especially in Gram-negative bacteria. Sensitivity limits The minimum inhibitory concentrations for amoxicillin/clavulanic acid correspond to the detection limits established by the European Committee on Microorganisms. For more details, see the Instructions attached to the drug. The prevalence of resistance of individual species is geographically and temporally dependent, and therefore it is advisable to obtain local information on antibiotic resistance before initiating therapy, especially in the case of severe infections. In cases where local rates of antibiotic resistance call into question the appropriateness of the drug for at least some types of infections, assistance should be sought from appropriate specialists. Pharmacokinetics Amoxicillin and clavulanic acid are completely soluble in water at physiological pH levels. Both components are quickly and well absorbed after taking the drug orally. Their absorption is improved if the drug is taken immediately before meals. When taken orally, the bioavailability of amoxicillin and clavulanic acid reaches approximately 70%. The plasma concentration profiles of both components are similar, with the time to peak concentration (Tmax) for each substance being approximately one hour. When groups of healthy volunteers took the 875 mg/125 mg combination tablet twice daily on an empty stomach, maximum serum concentrations (Cmax) were 11.64 to 2.78 mcg/mL for amoxicillin and 2.18 to 0.99 mcg/mL for clavulanic acid. Time to maximum serum concentration (Tmax) was 1.5 hours (range 1.0-2.5) for amoxicillin and 1.25 hours (range 1.0-2.0) for clavulanic acid. The average 1/p values were 1.19–0.21 h for amoxicillin and 0.96–0.12 h for clavulanic acid. Serum concentrations of amoxicillin and clavulanic acid achieved by oral administration of the combination product are similar to those obtained by oral administration of equivalent doses of amoxicillin or clavulanic acid alone. About 25% of the total plasma clavulanic acid content and 18% of the total plasma amoxicillin content is in a protein-bound state. The apparent volume of distribution is about 0.3-0.4 L/kg for amoxicillin and about 0.2 L/kg for clavulanic acid. After intravenous administration, amoxicillin and clavulanic acid are found in the gallbladder, abdominal wall tissue, skin, adipose tissue, muscle tissue, synovial and peritoneal fluids, bile and pus. Amoxicillin penetrates into the cerebrospinal fluid only to a small extent. Amoxicillin, like most penicillins, passes into breast milk. Trace amounts of clavulanic acid are also detected in breast milk (see Pregnancy and Breastfeeding). Both amoxicillin and clavulanic acid cross the placental barrier. Amoxicillin is partially excreted in the urine in the form of inactive penicillic acid in volumes equivalent to no more than 10-25% of the original dose. Clavulanic acid is intensively metabolized in the human body, excreted in urine and feces, and also in the form of carbon dioxide in exhaled air. The main route of elimination of amoxicillin is the kidneys, while clavulanic acid is eliminated from the body through renal and extrarenal mechanisms. The amoxicillin/clavulanic acid combination has an average elimination half-life of approximately one hour and an average total clearance of approximately 25 L/h in healthy individuals. Approximately 60-70% of amoxicillin and approximately 40-65% of clavulanic acid are excreted unchanged in the urine in the first 6 hours after a single dose of amoxicillin/clavulanic acid 250 mg/125 mg or 500 mg/125 mg tablets. The urinary excretion rate over a 24-hour period is 50-85% for amoxicillin and 27-60% for clavulanic acid. The maximum amount of clavulanic acid is excreted in the first two hours after taking the drug. Age The half-life of amoxicillin is similar in children aged three months to two years, older children and adults. For elderly people, the dose is selected with caution due to the possible decrease in renal function and, if necessary, renal function is regularly checked. Gender The pharmacokinetics of amoxicillin or clavulanic acid does not depend on the gender of the patient. Impaired renal function The total plasma clearance of amoxicillin and clavulanic acid decreases in proportion to the decrease in renal function. The decrease in clearance is more pronounced for amoxicillin than for clavulanic acid, since the proportion of amoxicillin excreted by the kidneys is higher. In renal failure, doses are adjusted to avoid excessive accumulation of amoxicillin while maintaining adequate levels of clavulanic acid (see Dosage and Administration). Liver failure In patients with liver failure, the drug is prescribed with caution and liver function is regularly monitored.
Indications for use Amoxiclav 2X is indicated for the treatment of the following infections in adults and children: - acute bacterial sinusitis (properly diagnosed); - acute otitis media; - exacerbation of chronic bronchitis (properly diagnosed); — community-acquired pneumonia; - cystitis; - pyelonephritis; - infections of the skin and soft tissues, in particular inflammation of the subcutaneous tissue, wounds from animal bites, severe tooth abscess with widespread inflammation of the subcutaneous tissue; - infections of bones and joints, in particular osteomyelitis. Official guidelines for the appropriate use of antibacterial agents should be considered.
Contraindications Hypersensitivity to the active or excipients of the drug, as well as to any penicillins. History of severe immediate hypersensitivity reactions (eg, anaphylaxis) to other beta-lactam drugs (eg, cephalosporins, carbapenems or monobactams). History of jaundice or other liver damage associated with amoxicillin/clavulanic acid use.
Doses and route of administration Doses reflect the content of amoxicillin/clavulanic acid. When choosing a dose for the treatment of specific infections, the following factors must be taken into account: - suspected pathogens and their possible susceptibility to antibacterial drugs; — severity and localization of infection; - age, weight and kidney function, as follows. The use of other dosage forms of the drug (for example, with higher doses of amoxicillin and/or with a different amoxicillin/clavulanic acid dose ratio) is considered as needed. When taking the drug 1 tablet 2 times a day in the doses recommended below, adults and children weighing > 40 kg will receive a total daily dose equal to 1750 mg amoxicillin / 250 mg clavulanic acid, and when taking 1 tablet 3 times a day - 2625 mg amoxicillin / 375 mg clavulanic acid. If a higher daily dose of amoxicillin is required, it is recommended to select a different dosage form of the drug to avoid taking excessively high daily doses of clavulanic acid. The duration of therapy is determined by the response to treatment. Some infections (such as osteomyelitis) require longer treatment. The duration of treatment should not exceed 14 days without review (see information about long-term therapy in the section Special instructions and precautions). Adults and children weighing > 40 kg Recommended doses: - standard dose (for all indications) - one tablet twice a day; - higher dose (especially for the treatment of infections such as otitis media, sinusitis, lower respiratory tract and urinary tract infections) - one tablet three times a day. Elderly patients (>65 years) No dose adjustment is required. Patients with impaired renal function Patients with creatinine clearance more than 30 ml/min do not require dose adjustment. Amoxiclav 2x is not used to treat patients with creatinine clearance <30 ml/min, since recommendations for selecting the dose of the drug for these patients have not been developed. Patients with impaired liver function Use with caution. Monitor liver function regularly (see Contraindications and Special Instructions and Precautions). Children weighing less than 40 kg The drug Amoxiclav 2x is not intended for children weighing less than 40 kg. If the patient has forgotten to take the prescribed dose of the drug, it should be taken as soon as possible. In this case, the interval before taking the next dose after this should be more than 4 hours. Then you should return to the previous dosage regimen. Do not take a double dose.
Directions for use: For oral administration. Take immediately before meals to minimize potential gastrointestinal intolerance and optimize absorption of amoxicillin/clavulanic acid.
Special instructions and precautions Before starting drug therapy, a thorough medical history is taken for hypersensitivity reactions to penicillins, cephalosporins or other beta-lactam drugs. Serious and sometimes fatal hypersensitivity reactions (anaphylactoid reactions) have been observed during penicillin therapy. They are most likely to develop in patients with hypersensitivity reactions to penicillins and a history of atopy. If an allergic reaction develops, therapy with Amoxiclav 2x is stopped and other suitable antibacterial drugs are prescribed instead. In cases of proven susceptibility of infectious agents to amoxicillin, a transition from Amoxiclav 2x to amoxicillin is considered in accordance with official guidelines. This dosage form of the drug is not suitable for use if there is a high risk that the suspected pathogens have resistance due not to the production of beta-lactamases sensitive to the inhibition of clavulanic acid, but to changes in penicillin-binding proteins (including resistant S. pneumoniae). Patients with impaired renal function or those receiving high-dose therapy may develop seizures (see Side effects). Therapy with Amoxiclav 2X should be avoided if infectious mononucleosis is suspected, since after the use of amoxicillin against the background of this disease, the appearance of a measles-like rash was observed. Concomitant use of allopurinol during treatment with amoxicillin potentially increases the likelihood of allergic skin reactions. Long-term use of Amoxiclav 2X can lead to excessive proliferation of non-susceptible microorganisms. The development of generalized erythema with fever and pustule formation at the beginning of therapy is a potential symptom of acute generalized exanthematous pustulosis (AGEP). Such a reaction requires discontinuation of therapy with Amoxiclav 2X and is a contraindication to the subsequent use of amoxicillin. Treatment of patients with liver failure is carried out with caution. Hepatic adverse events were observed predominantly in men and elderly patients and are potentially associated with long-term treatment. These adverse events have been observed in very rare cases in children. In all patient groups, signs and symptoms usually occur during or shortly after treatment, but in some cases they do not appear until several weeks after stopping therapy. Usually they are reversible. Severe liver side effects may develop, extremely rarely with death. They have almost always been observed among patients with serious underlying diseases or concomitant medications that can damage the liver. Cases of antibiotic-associated colitis, observed during therapy with almost all antibacterial drugs, including amoxicillin, can range in severity from mild to life-threatening. It is therefore important to consider this diagnosis in patients with diarrhea during or after completion of any course of antibiotic therapy. If antibiotic-associated colitis develops, you should immediately stop therapy with Amoxiclav 2X, consult a doctor and begin appropriate treatment. In this situation, the use of drugs that inhibit peristalsis is contraindicated. During long-term therapy, periodic assessment of the functions of various organ systems, including the kidneys, liver and hematopoietic organs, is recommended. In rare cases, while taking the drug, an increase in prothrombin time was observed. When taking anticoagulants concomitantly, proper monitoring of coagulation parameters is mandatory. Dosage adjustments of oral anticoagulants may be necessary to achieve the desired level of anticoagulation. In patients with renal failure, dose adjustment is required according to the level of insufficiency (see Doses and Administration). In patients with reduced diuresis, crystalluria was observed in rare cases, mainly during parenteral therapy. During therapy with high doses of amoxicillin, adequate fluid intake and diuresis control are recommended to reduce the likelihood of amoxicillin-associated crystalluria. In patients with a catheter installed in the bladder, it is necessary to regularly monitor its patency. If it is necessary to assess urine glucose levels during treatment with amoxicillin, enzymatic methods with glucose oxidase should be used, since non-enzymatic methods sometimes give false-positive results. The presence of clavulanic acid in Amoxiclav 2X can cause nonspecific binding of IgG and albumin to red blood cell membranes, which can lead to false-positive Coombs test results. Cases of positive enzyme immunoassay (ELISA) results for Aspergillus have been observed in patients receiving the drug who were subsequently determined to be free of Aspergillus infections. Cross-reactions with non-Aspergillus polysaccharides and polyfuranoses have been observed in the Aspergillus ELISA test. Positive test results in patients taking Amoxiclav 2X should be interpreted with caution and confirmed by other diagnostic methods.
Pregnancy and breastfeeding Limited data on the use of the drug during pregnancy do not indicate an increased risk of congenital anomalies. In women with premature premature rupture of membranes, prophylactic treatment with amoxicillin/clavulanic acid was potentially associated with an increased risk of necrotizing enterocolitis in newborns. The use of the drug should be avoided during pregnancy unless the doctor considers treatment necessary. Both active ingredients are excreted into breast milk (there are no data on the effect of clavulanic acid on breastfed children). Breastfed children may develop diarrhea and fungal infections of the mucous membranes, which may require discontinuation of breastfeeding. Therapy with the drug during breastfeeding is possible only after assessing the balance of benefits and risks by the attending physician.
Impact on the ability to drive vehicles and operate machinery. Possible development of undesirable effects (for example, allergic reactions, dizziness, convulsions), potentially affecting the performance of these functions.
Side effects To classify the frequency of adverse reactions, the following categories were used: very common (> 1/10}, common (> 1/100 to < 1/10), uncommon (> 1/1,000 to < 1/100), rare (> 1/10,000 to < 1/1,000), very rare (< 1/10,000), unknown frequency (it is not possible to estimate from available data).The most common adverse reactions to the drug: diarrhea, nausea and vomiting Infectious and parasitic diseases Common: candidiasis of the skin and mucous membranes Unknown frequency: excessive proliferation of non-susceptible microorganisms Blood and lymphatic system disorders Rare: reversible leukopenia (including neutropenia), thrombocytopenia Unknown frequency: reversible agranulocytosis, hemolytic anemia, prolongation bleeding time and prothrombin time Immune system disorders Unknown: angioedema, anaphylaxis, serum syndrome, allergic vasculitis Nervous system disorders Uncommon: dizziness, headache. Unknown frequency: reversible hyperactivity, seizures. Gastrointestinal disorders Very common: diarrhea. Frequent: nausea (most often associated with oral use of high doses; gastrointestinal reactions can be minimized if the drug is taken at the beginning of meals), vomiting. Uncommon: indigestion. Unknown frequency: antibiotic-associated colitis (including pseudomembranous colitis and hemorrhagic colitis, see Special Instructions and Precautions), black tongue. Liver and biliary disorders Uncommon: increased levels of AST and/or ALT (moderate increases observed in patients treated with beta-lactam antibiotics, but the significance of these observations is unknown). Unknown frequency: hepatitis, cholestatic jaundice (these adverse events were observed during the use of other penicillins and cephalosporins, see Special instructions and precautions). Skin and subcutaneous tissue disorders If any allergic skin reaction develops, treatment is discontinued. Uncommon: skin rash, itching, urticaria. Rare: erythema multiforme. Unknown frequency: Stevens-Johnson syndrome, toxic epidermal necrolysis, bullous exfoliative dermatitis, acute generalized exanthematous pustulosis (AGEP). Renal and urinary tract disorders Unknown frequency: interstitial nephritis, crystalluria. If you notice any adverse reactions, including those not mentioned in these instructions, please notify your doctor.
Overdose It is possible to develop gastrointestinal symptoms, as well as disturbances in water and electrolyte balance. Cases of amoxicillin-associated crystalluria, sometimes leading to renal failure, have been observed. Patients with impaired renal function or those receiving high-dose therapy may develop seizures. Amoxicillin precipitates in urinary catheters, mainly after intravenous administration of large doses. It is necessary to regularly monitor the patency of catheters. For gastrointestinal symptoms, symptomatic treatment can be carried out, along with restoration of water and electrolyte balance. Amoxicillin and potassium clavulanate can be eliminated from the body by hemodialysis.
Interaction with other medicinal products and other types of interactions Oral anticoagulants Cases of increased international normalized ratio have been described in patients receiving maintenance therapy with acenocoumarol or warfarin during a prescribed course of amoxicillin. If co-administration of drugs is necessary, carefully monitor the prothrombin time or international normalized ratio at the beginning and after discontinuation of treatment with amoxicillin. Dose adjustment of oral anticoagulants may be required. Methotrexate Penicillins may decrease the excretion rate of methotrexate which could result in increased toxicity. Probenecid It is not recommended to use concomitantly probenecid, which reduces the secretion of amoxicillin by the renal tubules. The simultaneous use of probenecid with Amoxiclav 2X can lead to an increase and longer maintenance of the level of amoxicillin (but not clavulanic acid) in the blood.
Release form: Blisters (aluminium/aluminium foil), 5 or 7 tablets per blister, 2 blisters in a cardboard box.
Storage conditions Keep out of the reach of children. Store in a place protected from moisture at a temperature not exceeding 25C.
Shelf life: 2 years. Do not use after the expiration date stated on the package.
Conditions of release Dispensed by prescription.
Indications for use
Amoxiclav is an antibacterial drug; it is indicated for the treatment of infectious diseases caused by bacteria sensitive to penicillin and its analogues:
- infections of the upper respiratory tract and ENT organs (acute and chronic sinusitis, acute and chronic otitis media, retropharyngeal abscess, tonsillitis, pharyngitis);
- lower respiratory tract infections (acute bronchitis with bacterial superinfection, chronic bronchitis, pneumonia);
- urinary tract infections (eg, cystitis, urethritis, pyelonephritis);
- infections in gynecology;
- skin and soft tissue infections, including animal and human bites;
- infections of bone and connective tissue;
- biliary tract infections (cholecystitis, cholangitis);
- odontogenic infections.
Directions for use and dosage
Inside. The dosage regimen is set individually depending on the age, body weight, kidney function of the patient and the severity of the infection.
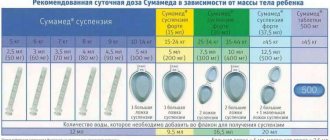
The daily dose of the suspension is 125 mg + 31.25 mg (to facilitate correct dosing, each package contains a dosing pipette graduated to 5 ml with a 0.1 ml division scale or a dosing spoon with a capacity of 5 ml, with ring marks in the cavity of 2.5 ml and 5 ml).
Newborns and children up to 3 months
30 mg/kg (amoxicillin) per day, divided into 2 doses (every 12 hours).
The dosage of the drug Amoxiclav with a dosage pipette is shown in the table. Calculation of single doses for the treatment of infections in newborns and children up to 3 months:
| Body weight (kg) | 2,0 | 2,2 | 2,4 | 2,6 | 2,8 | 3,0 | 3,2 | 3,4 | 3,6 | 3,8 | 4,0 | 4,2 | 4,4 | 4,6 | 4,8 |
| 250 mg+62.5 mg, ml (2 times a day) | 1,2 | 1,3 | 1,4 | 1,6 | 1,7 | 1,8 | 1,9 | 2,0 | 2,2 | 2,3 | 2,4 | 2,5 | 2,6 | 2,8 | 2,9 |
Children over 3 months
From 20 mg/kg for mild and moderate infections to 40 mg/kg for severe infections and lower respiratory tract infections, otitis media, sinusitis (according to amoxicillin) per day, divided into 3 doses (every 8 hours).
Dosing the drug Amoxiclav with a dosage spoon
Produced in the absence of a dosage pipette. Recommended doses of suspensions depending on the child’s body weight and the severity of the infection:
| Body weight (kg) | Age (approx.) | Mild/moderate course | Severe course | ||
| 125 mg+31.25 mg/ 5 ml | 250mg+62.5 mg/ 5ml | 125 mg+31.25 mg/ 5 ml | 250mg+62.5 mg/ 5ml | ||
| 5-10 | 3-12 months | 3 x 2.5ml (1/2 spoon) | 3 x 1.25 ml | 3 x 3.75 ml | 3 x 2 ml |
| 10- 12 | 1 - 2 g. | 3 x 3.75 ml | 3 x 2 ml | 3 x 6.25 ml | 3 x 3 ml |
| 12-15 | 2-4 years | 3 x 5 ml (1 spoon) | 3 x 2.5 ml (1/2 spoon) | 3 x 7.5 ml (1.5 spoons.) | 3 x 3.75 ml |
| 15-20 | 4-6 years | 3 x 6.25 ml | 3 x 3 ml | 3 x9.5 | 3 x 5 ml (1 spoon) |
| 20-30 | 6-10 years | 3 x 8.75 ml | 3 x 4.5 ml | — | 3 x 7 ml |
| 30-40 | 10-12 years | — | 3 x 6.5 ml | — | 3 x 9.5 ml |
| >40 | > 12 years | Amoxiclav tablets | |||
The maximum daily dose of amoxicillin is 6 g for adults and 45 mg/kg for children.
The maximum daily dose of clavulanic acid (in the form of potassium salt) is 600 mg for adults and 10 mg/kg body weight for children.
Instructions for preparing the suspension
Powder for preparing a suspension 125 mg + 31.25 mg: shake the bottle vigorously, add 85 ml of water in two additions (to the mark), shaking well each time until the powder is completely dissolved.
To prepare the suspension, it is recommended to dilute the powder with boiled water at room temperature.
It is recommended to place the finished suspension in the refrigerator.
It is not recommended to heat the suspension before use (it is necessary to bring the suspension to room temperature).
After taking the drug, it is recommended to rinse the dosage pipette with boiled water.
Analogues of the drug
If for some reason it is impossible to take Amoxiclav suspension for children, the antibiotic is replaced with another drug. Among the most popular analogues, the following should be highlighted:
- "Augmentin".
- "Flemoxin Solutab".
- "Ampisid."
- "Tazocin."
The drug "Augmentin" is a complete analogue of the drug "Amoxiclav". It is produced in the form of a powder for preparing a suspension for children. The differences between these two drugs are minimal. In a suitable dosage form, the antibiotic can be used from birth.
The drug "Flemoxin Solutab" contains amoxicillin as an active component. The drug is available in the form of soluble tablets. Doctors prescribe antibiotics to patients of any age.
"Ampiside" is a combination drug that contains ampicillin and sulbactam as an active component. The drug is produced in the form of a powder for the preparation of a solution for intramuscular and intravenous administration, as well as in the form of a powder for the preparation of a suspension, tablets. The drug is used to treat children, including premature babies.
The drug "Tazocin" is considered a good substitute for "Amoxiclav" in the clinical and pharmacological group. Its active ingredients are tazobactam and piperacillin. The medicine has a wide spectrum of antibacterial activity. Patients over two years of age are allowed to take it.
However, all of these analogues of Amoxiclav suspension can be purchased and used only after a doctor’s prescription.
Side effects
Negative side effects are possible when taking the drug:
- From the gastrointestinal tract - loss of appetite, diarrhea, nausea, vomiting. Very rarely, pain in the abdomen, increased activity of liver enzymes, impaired liver function, pseudomembranous colitis, hepatitis, cholestatic jaundice.
- From the hematopoietic and lymphatic system: thrombocytopenia, reversible leukopenia (rather rare), hemolytic anemia, eosinophilia, increased prothrombin time, pancytopenia (very rare).
- From the urinary system: crystalluria, interstitial nephritis (very rare);
- From the side of the central nervous system: headache, dizziness, insomnia, increased anxiety, hyperactivity; convulsions are possible in case of impaired renal function.
- From the skin: itching, erythematous rashes, in rare cases - exudative erythema, angioedema, allergic vasculitis, anaphylactic shock, generalized exanthematous pustulosis, Stephen-Jones syndrome.
- Other: candidiasis.
Drug interactions
When Amoxiclav is used simultaneously with glucosamines, aminoglycosides, laxatives and antacids, slow absorption is observed; when taken together with ascorbic acid, metabolism increases.
Medicines that block tubular secretion cause an increase in amoxicillin, while the simultaneous use of methotrexate increases the toxicity of the latter.
Simultaneous use with disulfiram is strictly contraindicated. Particular care should be taken when using Amoxiclav and anticoagulants simultaneously (this may cause prolongation of prothrombin time.
Interaction with probenecid increases the serum concentration of the antibiotic and reduces its excretion from the body. Amoxiclav, like other antibacterial drugs, reduces the effectiveness of oral contraceptives.
Contraindications
Contraindications to the use of Amoxiclav:
- hypersensitivity to any of the components of the drug;
- history of hypersensitivity to penicillins, cephalosporins and other β-lactam antibiotics;
- a history of indications of cholestatic jaundice and/or impaired liver function caused by taking amoxicillin/clavulanic acid;
- Infectious mononucleosis;
- lymphocytic leukemia
Features of use
Before starting therapy with Amoxiclav 457 mg in suspension, it is necessary to accurately determine the presence of a history of hypersensitivity reactions to penicillins, cephalosporins or other allergens. If allergic reactions occur, treatment should be discontinued.
If the infection is proven to be caused by microorganisms sensitive to amoxicillin, the possibility of switching from the amoxicillin/clavulanic acid combination to amoxicillin should be considered according to official recommendations.
Amoxiclav 400 mg + 57 mg suspension should not be used in cases of high risk that pathogens are resistant to β-lactams, and is not used for the treatment of pneumonia caused by penicillin-resistant strains of S. pneumoniae.
Children with impaired renal function or those receiving high doses may experience seizures.
Amoxiclav 400 mg + 57 mg should not be prescribed if infectious mononucleosis is suspected, since cases of morbilliform rashes have been observed with the use of amoxicillin for this pathology.
Long-term use of the drug can cause excessive growth of insensitive microflora and the development of pseudomembranous colitis of varying severity. In the presence of severe persistent diarrhea after the use of antimicrobial agents, it is important to ensure that it is not associated with this pathology. Drugs that suppress peristalsis are contraindicated. If colitis occurs, treatment should be stopped immediately and consult a doctor.
With long-term use, superinfection with resistant bacteria and fungi (Pseudomonas spp, Candida albicans) may occur, which requires discontinuation of therapy.
During long-term therapy, it is recommended to periodically assess organ functions, including renal, hepatic, and hematopoietic functions.
Declaration of Conformity
Overdose
There are no reports of life-threatening side effects due to an overdose of Amoxiclav. Symptoms of overdose include gastrointestinal disorders (abdominal pain, diarrhea, vomiting) and water and electrolyte imbalance. There have been reports of crystalluria caused by amoxicillin, which, in some cases, led to the development of renal failure.
Convulsions may develop in patients with renal failure or in patients receiving high doses of the drug.
In case of overdose, the patient should be under medical supervision and treatment should be symptomatic.
In case of overdose of the drug, gastric lavage and taking adsorbents (activated carbon) are recommended.
Amoxicillin and clavulanic acid are eliminated by hemodialysis.
special instructions
Like any other antibiotic, Amoxiclav 125 mg suspension for children has a number of individual properties that must be taken into account not only by the doctor, but also by the patient. During course therapy with the drug, the condition of all organs and hematopoietic systems, kidneys and liver of the patient is monitored.
In order to reduce the likelihood of undesirable manifestations from the gastrointestinal tract, it is recommended to take Amoxiclav suspension while consuming food. For patients with severe kidney pathologies, dosage regimen adjustments will be required.