Release form and composition
Dosage forms of Indapamide retard:
- extended-release tablets, film-coated: round, biconvex; the core on a cross section is white or almost white; depending on the manufacturer, the casing can be white or white with a grayish or light brown tint (in a contour pack there are 10, 15, 20 or 30 pcs., in a cardboard box there are 1–6, 8 or 10 packs - depending on the manufacturer; in polymer container 10, 20, 30, 40, 50 or 100 pcs., 1 container in a cardboard box);
- controlled-release tablets, film-coated: round, biconvex, almost white or white (10 pcs in a strip pack, 2-9 packs in a cardboard box; 15 pcs in a strip pack, 2, 4 in a cardboard box) or 6 packs).
Composition of 1 film-coated, extended-release tablet:
- active ingredient: indapamide – 1.5 mg;
- auxiliary components in the core (depending on the manufacturer): hypromellose, colloidal silicon dioxide (Aerosil), lactose monohydrate (milk sugar), magnesium stearate (ALSI Pharma), or hypromellose, magnesium stearate, microcrystalline cellulose, polyvinylpyrrolidone (OZONE), or hypromellose (hydroxypropyl methylcellulose), lactose monohydrate (milk sugar), magnesium stearate, povidone (low molecular weight polyvinylpyrrolidone), colloidal silicon dioxide (Tathimpharmaceuticals), or hypromellose (hydroxypropyl methylcellulose), copovidone, lactose monohydrate (milk sugar), microcrystalline cellulose, Kollidon SR (povidone, colloidal silicon dioxide anhydrous, polyvinyl acetate, sodium lauryl sulfate), magnesium stearate, colloidal silicon dioxide anhydrous (Canonpharma Production);
- shell (depending on the manufacturer): hypromellose, lactose monohydrate, polyethylene glycol, titanium dioxide (ALSI Pharma), or titanium dioxide, macrogol-4000, hypromellose, talc (OZONE), or macrogol 4000, polyvinyl alcohol, titanium dioxide, talc (Tathimpharmpreparaty , Kanonpharma Production).
Composition of 1 film-coated controlled-release tablet:
- active ingredient: indapamide – 1.5 mg;
- auxiliary components (core): hypromellose, mannitol, povidone K-90, colloidal silicon dioxide, magnesium stearate;
- shell: opadry II white (hypromellose, titanium dioxide, lactose monohydrate, macrogol, triacetin).
Pharmacological properties
Pharmacodynamics
The active substance of the drug, indapamide, is a thiazide-like diuretic with a long-lasting and moderately potent hypotensive effect. It has saluretic and diuretic effects associated with inhibition of active reabsorption of sodium, chlorine, and hydrogen ions in the cortical segment of the ascending loop of Henle and in the proximal tubules, which is accompanied by an increase in the amount of urine excreted and increased excretion of sodium, chlorine, potassium and magnesium ions by the kidneys.
Indapamide improves the elasticity of the walls of large arteries, reduces the tone of the smooth muscles of the arteries and reduces the total peripheral vascular resistance (TPVR) due to a decrease in the reactivity of the vascular wall to angiotensin II and norepinephrine, an increase in the synthesis of prostaglandins with vasodilatory activity, and inhibition of the flow of calcium ions into the smooth muscle vascular walls. The drug helps reduce hypertrophy of the left ventricle of the heart.
When indapamide is used in therapeutic doses, it does not affect the concentration of lipids in the blood plasma (high-density lipoproteins, low-density lipoproteins, triglycerides), and does not affect carbohydrate metabolism (including with concomitant diabetes mellitus).
After a single dose of indapamide, the antihypertensive effect persists for 24 hours, while a moderate diuretic effect is observed. With regular use of the drug, a stable hypotensive effect develops at the end of the first or beginning of the second week of therapy.
Pharmacokinetics
- absorption: after oral administration, indapamide is quickly and completely absorbed from the gastrointestinal tract; bioavailability – 93%. Eating slightly slows down the rate of absorption, but does not affect the completeness of absorption. The maximum plasma concentration (Cmax) is achieved 12 hours after oral administration of a single dose. With subsequent doses, fluctuations in the plasma concentration of the substance in the intervals between doses decrease;
- distribution: widely distributed in the body; 79% binds to blood plasma proteins; has the ability to pass through the blood-brain barrier and the placenta, and penetrates into breast milk. Equilibrium concentration (Css) is achieved after one week of regular use of the drug. Upon repeated administration, accumulation of the substance is not observed;
- metabolism and excretion: indapamide is metabolized in the liver; half-life (T1/2) – from 14 to 24 hours (average 18 hours); excreted mainly in the form of inactive metabolites: with urine - up to 80%, with feces - about 20%. 5% is excreted unchanged.
In patients with renal failure, pharmacokinetic parameters do not change.
Use of the drug during pregnancy and breastfeeding
As a rule, diuretics are not used during pregnancy.
These drugs cannot be used to treat physiological edema during pregnancy, because they can cause fetoplacental ischemia and can lead to impaired fetal development. The use of Indapamide retard is contraindicated during pregnancy.
Indapamide is excreted in breast milk. The use of Indapamide retard is contraindicated during breastfeeding.
If treatment with Indapamide retard is necessary during lactation, then breastfeeding must be stopped.
Contraindications
Absolute:
- severe forms of renal dysfunction (creatinine clearance below 30 ml/min);
- hypokalemia;
- lactose intolerance, lactase deficiency, glucose-galactose malabsorption;
- severe liver dysfunction (including hepatic encephalopathy);
- simultaneous use with drugs that prolong the QT interval (electrical systole of the heart);
- acute cerebrovascular accident;
- pregnancy, breastfeeding period;
- age under 18 years;
- increased individual sensitivity to indapamide, other sulfonamide derivatives or auxiliary components of the drug.
Relative (diseases/conditions in which the use of Indapamide retard requires caution):
- moderate renal and/or liver dysfunction;
- hyperparathyroidism;
- hyponatremia and other water and electrolyte balance disorders;
- increased QT interval on the electrocardiogram;
- diabetes mellitus in the stage of decompensation;
- hyperuricemia (especially accompanied by urate nephrolithiasis and gout).
What to replace with: analogues
Instructions for use of "Indapamide Retard" note: the product is based on indapamide. The following drugs were created using the same compound:
- "Ravel."
- "Indap".
- "Arifon".
- "Arifon Retard."
All of them are to some extent substitutes for the composition described.
If it is impossible to purchase a drug recommended by a doctor, the issue of replacement must be previously agreed upon with the attending physician. Quite often, doctors advise stopping at Arifon Retard. Indapamide is the active component of this drug, which in many of its parameters is similar to the one under consideration. However, an important difference for many is the cost of the product. If the medication described above in pharmacies costs on average from 30 to 150 rubles, then the price for a package of a popular analogue exceeds 300 rubles.
It is impossible to say for sure which is better - Indapamide or Arifon Retard. The first product is produced by several companies and has an affordable price. The second drug is a French development. It was he who appeared on the market first. The name of the medicine is patented, and the right to manufacture it belongs to Servier. Since medications for high blood pressure have to be used in long courses, a foreign product is not available to everyone. If you need to save money, you should check with your doctor to determine which is better for a particular patient - Arifon Retard or Indapamide. If the doctor confirms that the effect is equal and the likelihood of side effects is the same, the patient can choose the product that fits better into the family budget.
Instructions for use of Indapamide retard: method and dosage
The tablets are taken orally, swallowed whole, with sufficient liquid, preferably in the morning, regardless of meals.
The recommended dose of Indapamide retard is 1.5 mg (1 tablet) per day.
If the desired antihypertensive effect is not achieved after 4–8 weeks of therapy, the dose of the drug should not be increased (due to the risk of increasing the frequency of adverse reactions without enhancing the therapeutic effect). In this case, it is recommended to use another antihypertensive agent that is not a diuretic.
If it is necessary to start therapy using two different drugs, Indapamide retard should be taken in the same dose - 1 tablet in the morning, 1 time per day.
Rules of use
The manufacturer recommends taking the tablets orally. No chewing required. Patients who took the drug noted that the process of use did not pose any particular difficulties, but for some it was problematic to maintain stability in the time of administration.
In reviews of Indapamide Retard, patients who used the tablets mentioned that they drank one capsule of the drug per day. The manufacturer recommends the same dosage in the accompanying documentation. In some cases, some adjustments are possible based on the individual characteristics of the situation - the doctor will give recommendations.
Standard dosage regimen: one capsule in the morning, daily at the same time. “Indapamide Retard” is washed down with plenty of clean boiled water without additives.
Side effects
Most of the adverse reactions (clinical and laboratory indicators) are dose-dependent.
The incidence of side effects from systems and organs in accordance with a special scale (> 0.1 – very often; 0.01–0.1 – often; 0.001–0.01 – infrequently; 0.0001–0.001 – rarely; < 0.0001 – extremely rare; with an uncertain frequency – if it is impossible to calculate the frequency from the available data):
- central nervous system: rarely – headache, dizziness, paresthesia, fatigue;
- circulatory and lymphatic systems: very rarely - agranulocytosis, leukopenia, thrombocytopenia, hemolytic anemia, aplastic anemia;
- cardiovascular system: very rarely - decreased blood pressure, arrhythmia;
- digestive system: infrequently – vomiting; rarely - constipation, nausea, dry mouth; very rarely - pancreatitis;
- urinary system: very rarely - renal failure;
- liver and biliary tract: very rarely - liver function disorders; with an unknown frequency - the development of hepatic encephalopathy in case of liver failure;
- skin: often – maculopapular rash; uncommon – hemorrhagic vasculitis; very rarely - urticaria and/or angioedema, Stevens-Johnson syndrome, toxic epidermal necrolysis; with an unknown frequency - worsening of the disease in the acute form of disseminated lupus erythematosus; cases of photosensitive reactions have been reported;
- laboratory parameters: very rarely – hypercalcemia; with an unknown frequency - hyponatremia, hypokalemia, hyperglycemia, hyperuricemia, hypochloremia, glucosuria, hypercreatininemia, increased concentration of urea nitrogen in the blood plasma, increased QT interval on the electrocardiogram.
Negative consequences
Although observations of those taking the pills have shown that in most cases the medication is well tolerated, reviews of Indapamide Retard contain references to various unpleasant effects that occurred during the use of the medication. Not all patients complained about them - many admit that they did not encounter any side effects at all. The manufacturer indicates in the accompanying documentation for the tablets that use is associated with the risk of the following reactions:
- nausea, weight loss, stool upset, epigastric pain, liver encephalopathy, pancreatitis, dry mucous membranes, hepatitis;
- asthenia, agitation, pain and dizziness, sleep disturbances, depression, weakness, muscle spasms, anxiety, tension and nervousness;
- cough, inflammation in the throat, nasal cavity;
- disturbance of rhythm and speed, severity of heartbeat, sharp decrease in blood pressure;
- kidney infection, failure of this organ;
- allergic reactions, including itching, urticaria, necrolysis;
- thrombocyto-, leukopenia, anemia, agranulocytosis.
Taking pills against the background of lupus erythematosus can cause an exacerbation of this pathological condition. There are isolated cases of hypersensitivity to light. It is possible that laboratory parameters may change when samples are received from the patient for testing. "Indapamide Retard" can cause a lack of potassium, sodium, chlorine in the blood, excess calcium, urea nitrogen, creatinine. It is possible to detect glucose in urine.
Overdose
In case of an overdose of Indapamide retard, the following symptoms may develop: nausea/vomiting, dizziness, confusion, drowsiness, convulsions, excessive decrease in blood pressure, respiratory depression, oliguria up to anuria, polyuria, development of hepatic coma (in patients with impaired liver function).
There is no specific antidote for indapamide. Initial treatment consists of gastric lavage and/or activated charcoal. Then symptomatic therapy and restoration of the body’s water and electrolyte balance are recommended.
What it will help with: arterial hypertension
"Indapamide Retard" is prescribed for high blood pressure. It is customary to speak of a pathological condition when the pressure value is persistently maintained above 140/90. In rare cases, such parameters are normal for a person and are explained by individual characteristics - then no special adjustments are required. If the condition causes discomfort, treatment is necessary. Currently, arterial hypertension is one of the most common health disorders, if we analyze the diagnoses of patients around the planet. The older the person, the higher the likelihood of such failures.
Sometimes hypertension is an independent disease, but there are cases of its development against the background of other health problems. High blood pressure can be caused by improperly functioning adrenal glands, kidneys, and neoplasms. Often pressure is provoked by worries and stress, although other reasons are possible. As a rule, health problems develop gradually. At first, the pressure rises occasionally and insignificantly. If this is just a response to an external factor, then it is too early to talk about pathology. When blood pressure becomes persistently elevated, hypertension is diagnosed.
special instructions
During treatment with Indapamide retard, it is necessary to systematically monitor the concentration of sodium, potassium and magnesium ions in the blood plasma due to the possible occurrence of water and electrolyte balance disorders, check the pH level, the concentration of uric acid, glucose and residual nitrogen.
Patients taking laxatives, cardiac glycosides, elderly patients, as well as patients with aldosteronism syndrome are advised to regularly monitor the concentration of potassium and creatinine ions.
The first measurement of potassium ions in the blood must be carried out within 7 days from the start of treatment.
In patients with impaired liver function, the use of thiazide-like diuretics increases the risk of developing hepatic encephalopathy, especially with water-salt balance disorders. In this case, you should immediately stop taking Indapamide retard.
The most careful monitoring of potassium levels in the blood is necessary in patients with heart failure, ischemic heart disease (coronary artery disease), liver cirrhosis (especially with ascites or edema - due to the risk of developing metabolic alkalosis). The high-risk group also includes patients with an increased QT interval on the electrocardiogram. Hypokalemia is a predisposing factor in the development of severe arrhythmias, especially torsades de pointes, which can be fatal.
An increase in plasma calcium concentration during the use of Indapamide retard may be observed due to previously undiagnosed hyperparathyroidism.
In diabetes mellitus, it is important to carefully monitor blood glucose levels, especially if there is hypokalemia.
At the initial stage of treatment, kidney function should be checked. Patients with impaired renal function need to replenish the circulating blood volume (circulating blood volume). Significant dehydration can cause the development of acute renal failure.
In patients suffering from gout, while taking indapamide, the frequency of attacks may increase and/or the course of gout may worsen.
If it is necessary to use ACE inhibitors (angiotensin-converting enzyme) in patients with arterial hypertension and hyponatremia, stop taking indapamide 3 days before starting ACE inhibitors (with the possibility of subsequently resuming the diuretic) or prescribe initially low doses of ACE inhibitors.
Before testing the function of the parathyroid glands, you should stop taking the drug.
Indapamide retard can give a positive result during doping control in athletes.
If photosensitivity reactions develop during the use of thiazide-like diuretics, drug therapy should be discontinued. If treatment must be continued, it is recommended to protect the skin from sunlight or artificial ultraviolet rays.
Impact on the ability to drive vehicles and complex mechanisms
The substances that make up Indapamide retard do not cause disturbances in psychomotor reactions. But due to the development of individual reactions in some patients to a decrease in blood pressure (especially at the initial stage of treatment or with concomitant therapy with other antihypertensive drugs), caution must be exercised when driving vehicles and performing all types of work associated with an increased risk of accidents.
How to notice?
The most significant manifestation of hypertension is blood pressure above 140/90. You can check the indicators yourself at home if you purchase a tonometer.
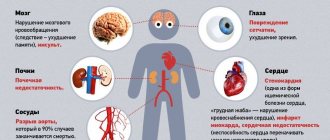
High blood pressure may be indicated by spots appearing before the eyes. Sometimes you feel dizzy and have a headache, and your vision is blurred. As a rule, the progress of the disease is associated with failures in the functionality of various internal organs. People suffering from high blood pressure are characterized by swelling, suffocation and shortness of breath are possible. Sometimes symptoms occur that suggest heart failure.
It is customary to distinguish three main degrees of hypertension. If the indicators vary within 159/99, they speak of the first degree. Progress is indicated by raising the parameters to 179/109. In the third degree, the pressure is above 180/110.
An isolated increase in systole only is possible when the first parameter exceeds 149, and diastole varies within 90.
There are several known reasons for this condition. If the disease is primary, in most cases it is not at all possible to determine why the pathology developed. It is known that the likelihood of experiencing high hypertension is higher if there are hypertensive patients among close relatives. Hypertension is more often observed in people over 50 years of age, as well as people who have bad habits, primarily smoking. Hypertension can be triggered by low mobility in everyday life, excess salt intake, endocrine diseases, diabetes mellitus, and frequent exposure to stress factors.
Secondary hypertension is often observed in diseases of the kidneys, blood vessels supplying these organs, and adrenal glands. There is a high probability of high blood pressure due to tumor processes and impaired blood dynamics. Lesions of the vascular system that cause hypertension are divided into congenital and acquired. Both types can equally cause higher than normal blood pressure. Another possible cause is obstructive sleep apnea. Sometimes hypertension is caused by taking medications (more often hormonal or temperature-lowering).
For impaired renal function
Indapamide retard is contraindicated for use in severe renal impairment (creatinine clearance below 30 ml/min).
With normal renal function during the period of use of thiazide-like diuretics, temporary renal failure observed at the beginning of treatment usually passes without consequences. In patients with impaired renal function, the condition may worsen.
Indapamide retard is fully effective only with normal or slightly reduced (in adults, plasma creatinine concentration below 220 µmol/l or 25 mg/l) renal function. A marked decrease in circulating blood volume can cause an increase in the concentration of urea and creatinine in the blood (due to a decrease in glomerular filtration rate), which can lead to the development of acute renal failure.
What's in pharmacies?
"Indapamide Retard" is available in tablet form. Capsules are packed in blisters. One blister contains 10-15 tablets (depending on the release form). The manufacturer places instructions for use and three blisters for 10 capsules or two for 15 capsules in a cardboard box. The name of the medicine, manufacturer, expiration date and production date, rules for dispensing the drug, and the exact number of tablets inside are indicated on the outside.
It is unacceptable to use Indapamide Retard after the expiration date. The product should be stored in a dark room at temperatures up to 25 degrees. If storage conditions are violated, the medicine should not be eaten. You need to choose a place to store it where children do not have access.
Drug interactions
The use of Indapamide retard simultaneously with certain drugs can lead to the development of the following effects:
- Lithium preparations: increased concentration of lithium in the blood plasma (with signs of overdose) due to decreased excretion. If simultaneous use with indapamide is necessary, it is important to monitor plasma lithium levels;
- thiazide diuretics, piretanide, furosemide, bumetanide, xipamide and other diuretics that can cause hypokalemia: not recommended to be combined with indapamide;
- antiarrhythmic drugs of subgroup IA (disopyramide, hydroquinidine, quinidine); class III antiarrhythmic drugs (ibutilide, dofetilide, amiodarone) and sotalol; some neuroleptics (phenothiazines - trifluoperazine, thioridazine, levomepromazine, cyamemazine, chlorpromazine; benzamides - tiapride, sultopride, sulpiride, amisulpride; butyrophenones - haloperidol, droperidol); erythromycin for intravenous use, cisapride, bepridil, sparfloxacin, pentamidine, mizolastine, halofantrine, astemizole, vincamine for intravenous use, moxifloxacin: increased risk of developing polymorphic ventricular tachycardia of torsades de pointes (risk factor - hypokalemia). It is necessary to adjust the concentration of potassium in the blood serum before starting combination treatment. Also, during combination therapy, clinical monitoring, electrocardiographic analysis and monitoring of the level of electrolytes in the blood should be carried out;
- systemic nonsteroidal anti-inflammatory drugs (including selective cyclooxygenase 2 inhibitors), high doses of acetylsalicylic acid (more than 3 g per day): reducing the hypotensive effect of indapamide, increasing the risk of acute renal failure in patients with dehydration. It is necessary to compensate for the lack of fluid in the body at the beginning of treatment with indapamide and regularly check the functioning of the kidneys throughout the course of therapy;
- ACE inhibitors: against the background of sodium deficiency (especially in patients with renal artery stenosis), a sharp decrease in blood pressure and/or an increase in the likelihood of developing acute renal failure is possible;
- drugs that cause hypokalemia (mineralocorticosteroids for systemic use, glucocorticosteroids, amphotericin B for intravenous use, tetracosactide, laxatives that stimulate intestinal motility): increased risk of hypokalemia (due to additive effects);
- baclofen: increased hypotensive effect of indapamide;
- cardiac glycosides: increased likelihood of developing hypokalemia, predisposing to the toxic effects of cardiac glycosides;
- potassium-sparing diuretics (triamterene, spironolactone, amiloride): increased risk of developing hypokalemia or hyperkalemia (especially in patients with renal failure and/or diabetes mellitus);
- Metformin: increased likelihood of metformin-induced lactic acidosis. Renal dysfunction associated with diuretic use may be a predisposing factor;
- iodine-containing contrast agents (in high doses): increased risk of developing acute renal failure (against the background of diuretic-induced dehydration). It is necessary to perform rehydration before using iodine-containing substances;
- tricyclic antidepressants and antipsychotics: increased hypotensive effect of indapamide and increased likelihood of orthostatic hypotension (due to additive action);
- calcium salts: increased risk of hypercalcemia due to decreased renal excretion of calcium;
- cyclosporine and tacrolimus: an increase in the concentration of creatinine in the blood plasma with unchanged levels of cyclosporine in the circulating blood, even without a deficiency of sodium and/or fluid in the body;
- corticosteroids (mineral and glucocorticosteroids) and tetracosactide (when administered systemically): reducing the hypotensive effect of indapamide due to fluid and sodium retention in the body caused by corticosteroids.
Clinical trial results
It has been reliably established that the described antihypertensive drug in its biochemical properties is very close to the behavior model of thiazide diuretics, that is, when it enters the body, it stimulates inhibition of reverse absorption with the participation of sodium ions (we are talking about the cortical segment of the so-called nephron loop). Long-term observations of the progress of monotherapy based on this medication (with a dosage that excludes a targeted increase in urination) made it possible to identify regular demonstrations of the desired result over time periods of 24 hours. In other words, even with a decrease in the recommended daily intake, indapamide has a targeted effect on vascular smooth muscle, which leads to suppression of characteristic symptoms and normalization of blood pressure. When the optimal dose was exceeded, almost all patients experienced a sharp development of side dynamics against the background of stabilization of the already obtained positive indicators.