Release form, composition and packaging
Tablets from white to white with a yellowish tint, round, biconvex, chamfered, with a score and the inscription “LAMISIL 250” (in a circle) on one side; with a smooth or slightly rough surface.
Active ingredient: terbinafine (in the form of hydrochloride) - 1 tablet. 250 mg.
Excipients: magnesium stearate, colloidal anhydrous silicon dioxide, methylhydroxypropylcellulose, microcrystalline cellulose, sodium starch glycolate.
7 pcs. - blisters (1) - cardboard packs. 14 pcs. - blisters (1) - cardboard packs. 14 pcs. - blisters (2) - cardboard packs.
pharmachologic effect
Antifungal drug.
Terbinafine is an allylamine that has a broad spectrum of action against fungi that cause diseases of the skin, hair and nails, incl. dermatophytes such as Trichophyton (for example, Trichophyton rubrum, Trichophyton mentagrophytes, Trichophyton verrucosum, Trichophyton tonsurans, Trichophyton violaceum), Microsporum (for example, Microsporum canis), Epidermophyton floccosum, as well as yeasts of the genus Candida (for example, Candida albicans) and Pityrosporum.
In low concentrations, terbinafine has a fungicidal effect against dermatophytes, molds and some dimorphic fungi. Activity against yeast fungi, depending on their type, can be fungicidal or fungistatic.
Terbinafine specifically inhibits the early stage of sterol biosynthesis in the fungal cell. This leads to ergosterol deficiency and intracellular accumulation of squalene, which causes the death of the fungal cell. Terbinafine works by inhibiting the enzyme squalene epoxidase in the cell membrane of the fungus. This enzyme does not belong to the cytochrome P450 system.
When Lamisil is administered orally, concentrations of the drug are created in the skin, hair and nails, providing a fungicidal effect.
Pharmacokinetics
Suction
After oral administration, terbinafine is well absorbed (>70%); The absolute bioavailability of terbinafine due to the “first pass” effect is approximately 50%.
After a single oral dose of terbinafine at a dose of 250 mg, its Cmax in plasma is reached after 1.5 hours and is 1.3 mcg/ml. With continuous use of terbinafine, its Cmax increased by an average of 25% more than with a single dose; AUC increased 2.3 times. Based on the increase in AUC, the effective T1/2 (30 hours) can be calculated. Food intake has a slight effect on the bioavailability of the drug (AUC increases by less than 20%), therefore no dose adjustment of Lamisil is required when taken simultaneously with food.
Distribution
Terbinafine is highly bound to plasma proteins (99%). Quickly penetrates the dermal layer of the skin and concentrates in the lipophilic stratum corneum. Terbinafine also penetrates the secretions of the sebaceous glands, which leads to the creation of high concentrations in the hair follicles, hair and skin rich in sebaceous glands. Terbinafine has also been shown to penetrate the nail plates in the first few weeks after initiation of therapy.
Metabolism and excretion
Terbinafine is metabolized quickly and substantially with the participation of at least 7 isoenzymes of cytochrome P450, with the main role being played by the isoenzymes CYP2C9, CYP1A2, CYP3A4, CYP2C8 and CYP2C19. As a result of the biotransformation of terbinafine, metabolites are formed that do not have antifungal activity and are excreted primarily in the urine.
Pharmacokinetics in special clinical situations
There were no changes in Lamisil plasma Css depending on age.
In pharmacokinetic studies of a single dose of Lamisil in patients with concomitant renal impairment (creatinine clearance <50 ml/min) and liver disease, the possibility of reducing drug clearance by 50% was shown.
Effect of the drug
Lamisil has a wide spectrum of action. Only the activity of the drug against pathogens of dermatomycosis is clinically significant, which is the basis for determining the list of indications for use.
The effect of the medication applies to fungi that affect the skin, hair and nails. Low concentrations of terbinafine provide a fungicidal effect. This is how the drug acts on dermatophytes, molds and some dimorphic fungi. The effect on yeast fungi can be fungicidal or fugistatic - the type of activity depends on the type of microorganism.
Terbinafine provides a specific effect regarding sterols at the cellular level. As a result, their biosynthesis is suppressed at an early stage. Against this background, there is a lack of ergosterol, and squalene accumulates inside the cells, so the fungi are destroyed at the cellular level.
The effect of the drug is due to the fact that the enzyme squalene epoxidase is blocked. The object of this reaction is the membranes of fungal cells.
Lamisil tablets are used for oral administration, while the skin, hair and nails are provided with the required concentration of the drug to create a fungicidal effect.
When taking Lamisil internally, good absorption is observed, amounting to more than 70%. There is a first pass effect, so the level of absolute bioavailability is approximately 50%.
A single dose of one tablet ensures a maximum plasma concentration of 1.3 mcg/ml within 1.5 hours. If the drug is used continuously, this figure increases by an average of a quarter. The half-life takes about 30 hours.
There is a relationship between the bioavailability of the drug and food intake. It is insignificant, therefore adjustment of the dose taken with food is not required.
The binding of the drug to plasma proteins is significant and amounts to 99%. There is rapid penetration of terbinafine through the dermis and its concentration in the lipophilic stratum corneum. The substance also appears in the secretion of the sebaceous glands, which is why hair, hair follicles and skin accumulate it in high concentrations. During the first weeks of therapy, penetration of terbinafine into the nail plates is also observed.
Terbinafine is metabolized quickly. Cytochrome P450 isoenzymes are involved in this process. Biotransformation of the substance leads to the formation of metabolites that do not have antifungal activity. They are excreted in the urine.
If renal function is impaired and creatinine clearance is less than 50 ml/min, the rate of drug elimination may be halved.
Indications for use
List of indications:
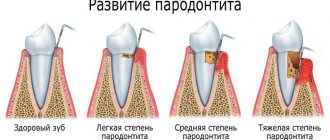
- onychomycosis caused by dermatophyte fungi;
- mycoses of the scalp;
- fungal infections of the skin - treatment of dermatomycosis of the trunk, legs, feet, as well as yeast infections of the skin caused by fungi of the genus Candida (for example, Candida albicans) - in cases where the localization, severity or prevalence of the infection determine the advisability of oral therapy.
Unlike topical Lamisil, oral Lamisil is not effective for tinea versicolor.
Contraindications
Contraindicated in case of hypersensitivity to the components of the drug.
It is not recommended to prescribe Lamisil to patients with chronic or active liver disease. Before prescribing Lamisil tablets, it is necessary to determine whether the patient has pre-existing liver disease. Hepatotoxicity can occur in patients with or without pre-existing liver disease.
Patients prescribed Lamisil should be warned that it is necessary to immediately inform the attending physician if symptoms such as persistent nausea, lack of appetite, fatigue, vomiting, pain in the right hypochondrium, jaundice, dark urine or light-colored stool occur while taking the drug. .
If such symptoms occur, you should immediately stop taking the drug and conduct a liver function test.
Since the use of the drug in patients with impaired renal function (creatinine clearance <50 ml/min or serum creatinine concentration >300 µmol/l) has not been sufficiently studied, Lamisil is not recommended for use in this category of patients.
Reviews
A year ago, we persuaded my grandfather to go to a dermatologist because he had terrible nail fungus. The doctor prescribed Lamisil tablets and cream without interruption. Within six months, our feet became almost clean. Now we are finishing the treatment with a spray, because my grandfather is tired of applying cream. The most important thing in treatment is not to give up halfway!
— Anastasia, 34 years old
A few months ago I went to the pool, and as a result I managed to catch nail fungus. The dermatologist prescribed this medication for me. During the period of treatment, I began to have a severe headache, tinnitus, nausea and abdominal pain. The doctor prescribed another medicine. It’s a pity, of course, that my body does not accept this remedy, because reviews on the Internet about it are mostly only positive.
— Gregory, 45 years old
Personally, I was helped by Exifin tablets, the cost of which fluctuates around 700 rubles. However, first the dermatologist prescribed me Lamisil for my nail fungus. I never noticed any positive effect; I threw away 2,000 thousand rubles. There was a residue in my soul.
— Alena, 26 years old
Dosage
The duration of treatment depends on the indication and severity of the disease.
There are no data on the use of the drug in children under 2 years of age (whose body weight is usually less than 12 kg). In children over 2 years of age, Lamisil for oral administration is well tolerated.
Calculation of dosage based on the child’s :
- less than 20 kg - 62.5 mg;
- from 20 to 40 kg - 125 mg;
- more than 40 kg - 250 mg.
Adults are prescribed 250 mg 1 time/day.
Recommended duration of treatment:
- for dermatomycosis of the feet (interdigital, plantar or sock-type) - 2-6 weeks;
- for dermatomycosis of the trunk, legs - 2-4 weeks;
- for skin candidiasis - 2-4 weeks.
Complete disappearance of the manifestations of infection and complaints associated with it can occur only a few weeks after mycological cure.
The recommended duration of treatment for mycosis of the scalp is 4 weeks. Mycoses of the scalp are observed mainly in children.
For onychomycosis, the duration of effective treatment in most patients is from 6 to 12 weeks. For onychomycosis of the hands, in most cases, 6 weeks of treatment is sufficient. For onychomycosis of the feet, in most cases, 12 weeks of treatment is sufficient. Some patients who have a reduced rate of nail growth may require longer treatment.
The optimal clinical effect is observed several months after mycological cure and cessation of therapy. This is determined by the period of time required for a healthy nail to grow back.
There is no reason to assume that elderly patients require dosage adjustments or that they experience adverse reactions that differ from those of younger patients. When using the drug in tablet form in this age group, the possibility of concomitant liver or kidney dysfunction should be taken into account.
Instructions for use of Lamisil: method and dosage
Tablets Take orally 1 time per day. The dose and duration of therapy are prescribed by the doctor based on clinical indications and the patient’s condition. Recommended daily dosage: for adult patients – 1 tablet; for children weighing: up to 20 kg - ¼ tablet, from 20 to 40 kg - ½ tablet, above 40 kg - 1 tablet. The drug is well tolerated in children. Recommended duration of taking the tablets: mycosis of the scalp (more often observed in children) – 4 weeks; dermatomycosis of the feet (plantar, interdigital or sock-type) – from 2 to 6 weeks; dermatomycosis of the trunk and legs, skin candidiasis - 2-4 weeks; onychomycosis - from 6 to 12 weeks (on the hands - 6 weeks, on the feet - 12 weeks) or the period of growth of a new healthy nail (if the rate of nail growth is reduced, longer treatment may be required). In the absence of concomitant liver and/or kidney dysfunction, elderly patients do not require dose adjustment;
Cream for external use Apply a thin layer to a previously cleaned and dried affected area of the body and rub into the skin with light movements, capturing adjacent areas. When treating diaper rash in the interdigital spaces, under the mammary glands, between the buttocks and in the groin area, the use of gauze bandages is recommended, especially when applying the cream at night. The frequency of procedures and the treatment period depend on the clinical indications: ringworm of the trunk, legs, feet - 1 time per day for 1 week, for ringworm of the feet aggravated by keratinization, cracks, peeling and itching of the skin - 1-2 times a day for 2 weeks; skin candidiasis – 1-2 times a day for 1-2 weeks; for tinea versicolor – 1-2 times a day for 2 weeks. If there are no signs of effect after 1-2 weeks of therapy, you should consult a doctor to verify the diagnosis;
Spray for external use Apply by spraying onto previously cleaned and dried affected and adjacent areas of the body until the skin is thoroughly moisturized. Treatment period and frequency of spraying: for dermatomycosis of the feet, legs and torso – 1 week, 1 time per day; for tinea versicolor – 1 week, 2 times a day; for inguinal epidermophytosis, diaper rash - 1 week, 1 time per day.
Side effects
Lamisil is generally well tolerated, side effects are usually mild to moderate and transient. The following are adverse events that were observed during clinical trials or after the drug was marketed.
When assessing the frequency of side effects, the following gradations were used: very often (≥1/10), often (≥1/100, <1/10), sometimes (≥1/1000, <1/100), rarely (≥1/ 10,000, <1/100), very rare (<1/10,000), including isolated reports.
From the hematopoietic system: very rarely - neutropenia, agranulocytosis, thrombocytopenia, pancytopenia. In very rare cases, when using the drug, the development of qualitative or quantitative changes in blood cells (neutropenia, agranulocytosis, thrombocytopenia, pancytopenia) was observed. If qualitative or quantitative changes in blood cells develop, the cause of the disturbances should be established and consideration should be given to reducing the dose of the drug or, if necessary, discontinuing therapy with Lamisil.
From the immune system: very rarely - anaphylactoid reactions (including angioedema), cutaneous and systemic lupus erythematosus.
From the nervous system: often - headache; sometimes - disturbances in taste, including their loss (usually recovery occurs within a few weeks after stopping treatment). There are isolated reports of cases of long-term disturbances in taste. In some cases, while taking the drug, there was a decrease in food consumption, which leads to a significant decrease in body weight. Dizziness, paresthesia, and hypoesthesia were very rarely observed.
From the hepatobiliary system: rarely - hepatobiliary dysfunction (mainly cholestatic in nature), including very rare cases of severe liver failure (some fatal or requiring liver transplantation). In most cases where liver failure developed, patients had serious concomitant systemic diseases and the causal relationship of liver failure with Lamisil was questionable.
From the digestive system: very often - a feeling of fullness in the stomach, loss of appetite, dyspepsia, nausea, mild abdominal pain, diarrhea.
Dermatological reactions: very often - mild skin reactions (rash, urticaria); very rarely - serious skin reactions (including Stevens-Johnson syndrome), psoriasis-like skin rashes or exacerbation of psoriasis. Very rarely, cases of hair loss have been reported, although the cause-and-effect relationship of this phenomenon with taking the drug has not been established. If a progressive skin rash develops, treatment with Lamisil should be discontinued.
From the musculoskeletal system: very often - arthralgia, myalgia.
Other: very rarely - feeling of fatigue.
Side effects, overdose
The use of Lamisil is attractive because it is well tolerated, so in case of adverse reactions, their manifestation is mild or moderate. More often, therapy causes headache, a feeling of fullness in the stomach, loss of appetite, dyspepsia, diarrhea, nausea, and minor abdominal pain. Taste sensations may be impaired. There is a risk of arthralgia or myalgia.
Less commonly, side effects include hepatobiliary dysfunction. In rare cases, Lamisil causes agranulocytosis, neutropenia, thrombocytopenia, and severe liver failure. There is a risk of anaphylactoid reactions, cutaneous or systemic lupus erythematosus.
If the dose of the medicine is exceeded, it can cause headache, nausea, dizziness or pain in the epigastrium.
The first measures in this case should be gastric lavage and taking activated charcoal. In some cases, symptomatic or supportive treatment is required.
Drug interactions
Effect of other drugs on terbinafine
Plasma clearance of terbinafine can be accelerated by drugs that are metabolic inducers and suppressed by cytochrome P450 inhibitors. If it is necessary to use the above drugs and Lamisil simultaneously, an appropriate adjustment of the dosage regimen of the latter may be required.
Cimetidine may enhance the effect of terbinafine or increase its plasma concentration. Cimetidine reduces the clearance of terbinafine by 33%.
Rifampicin may weaken the effect of terbinafine or reduce its plasma concentration. Rifampin increases the clearance of terbinafine by 100%.
Effect of terbinafine on other drugs
Results from in vitro and healthy volunteer studies indicate that terbinafine has little potential to inhibit or enhance the clearance of most drugs metabolized by the cytochrome P450 system (e.g., terfenadine, triazolam, tolbutamide, or oral contraceptives), except those which are metabolized with the participation of CYP2D6.
Terbinafine does not affect the clearance of antipyrine or digoxin.
There have been reports of several cases of menstrual irregularities in patients taking Lamisil in conjunction with oral contraceptives, although the frequency of these disorders does not exceed the average frequency of such disorders in patients taking only oral contraceptives.
Terbinafine may enhance the effects of caffeine or increase its plasma concentration. Terbinafine reduces the clearance of caffeine when administered intravenously by 19%.
Terbinafine has been shown to inhibit CYP2D6-mediated metabolism in in vivo and in vitro studies. These data may be clinically significant for those drugs that are predominantly metabolized by this enzyme: tricyclic antidepressants, beta-blockers, selective serotonin reuptake inhibitors, class 1A, 1B, 1C antiarrhythmic drugs and MAO type B inhibitors, if the drug used At the same time, the drug has a small range of therapeutic concentrations.
Terbinafine reduces the clearance of desipramine by 82%.
Terbinafine may weaken the effect of cyclosporine and reduce its plasma concentration. Terbinafine increases the clearance of cyclosporine by 15%.
Compatibility with other drugs
During therapy with Lamisil, it is important to consider that medications taken concomitantly may lead to certain reactions.
It is possible to accelerate the plasma clearance of Lamisil if metabolic inducers are taken simultaneously. The opposite reaction is observed when cytochrome P450 inhibitors are used. In both cases, you may need to adjust your Lamisil dosage.
It is possible to increase the effectiveness of terbinafine or its plasma concentration with simultaneous use of cimetidine. In this case, there is a decrease in its clearance by a third.
When combining rifampicin and terbinafine, the clearance of the latter doubles.
If drugs that are metabolized by cytochrome P450 are taken simultaneously with Lamisil, their clearance may be slightly increased or suppressed. The combination of Lamisil with digoxin or antipyrine does not affect the clearance of the latter.
If the patient takes oral contraceptives, then in combination with antifungal therapy this can cause menstrual irregularities.
The use of Lamisil affects the clearance of caffeine, reducing it by almost 20% in the case of intravenous administration. With simultaneous use of desipramine, its clearance is reduced by more than 80%.
If drug metabolism is carried out with the participation of the CYP2D6 enzyme, then when combined with Lamisil it may be suppressed. This factor may have clinical significance if this enzyme is the predominant mechanism of metabolism. This feature is characteristic of tricyclic antidepressants, selective serotonin reuptake inhibitors, β-blockers, monoamine oxidase type B inhibitors, and antiarrhythmics belonging to class 1C. If it is necessary to simultaneously include such medications with Lamisil, the patient needs constant monitoring.
When Lamisil is combined with cyclosporine, the effect of the latter is weakened, and plasma concentrations may decrease. In this case, the clearance of the drug increases by 15%.
Combination with fluconazole can affect the maximum concentration of the drug in the blood, increasing it by more than 50%. A similar effect is observed when taking amiodarone or ketoconazole simultaneously.
special instructions
Terbinafine has been shown to inhibit metabolism mediated by the CYP2D6 enzyme. Therefore, it is necessary to constantly monitor patients receiving concomitant treatment with Lamisil with drugs that are predominantly metabolized with the participation of this enzyme (such as tricyclic antidepressants, beta-blockers, selective serotonin reuptake inhibitors, class 1C antiarrhythmic drugs and MAO inhibitors if used simultaneously the drug has a small range of therapeutic concentrations.
The effect of Lamisil on the ability to drive vehicles and operate machinery has not been studied. If dizziness develops during drug therapy, patients should not drive vehicles and/or operate machinery.
Pregnancy and lactation
Data from experimental studies do not suggest the presence of adverse effects on fertility and toxic effects on the fetus. Since clinical experience with Lamisil during pregnancy is very limited, the drug should not be used during pregnancy unless the expected benefit of therapy outweighs the potential risk.
Terbinafine is excreted in breast milk, so women receiving Lamisil by mouth should not breast-feed.
For liver dysfunction
It is not recommended to prescribe Lamisil to patients with chronic or active liver disease. Before prescribing Lamisil tablets, it is necessary to determine whether the patient has pre-existing liver disease. Hepatotoxicity can occur in patients with or without pre-existing liver disease.
Patients prescribed Lamisil should be warned that it is necessary to immediately inform the attending physician if symptoms such as persistent nausea, lack of appetite, fatigue, vomiting, pain in the right hypochondrium, jaundice, dark urine or light-colored stool occur while taking the drug. . If such symptoms occur, you should immediately stop taking the drug and conduct a liver function test.
Reviews about Lamisil
Reviews about Lamisil are mostly positive. Despite the relatively high cost of the drug, it is considered an effective treatment for nail fungus. Within a few days after the start of treatment, the patients' condition improved. The desired result can be most quickly achieved by combining tablets with the use of Lamisil cream or spray. However, in the treatment of nail fungus and other fungal diseases, the use of only local forms of the drug is also successful.
There are also a few negative reviews about the lack of effectiveness of the drug.