Manufacturer Dimia
The hormonal drug is produced by a world-famous product. This company has been working in the pharmaceutical field since 1901, the same year it was founded. The company is based in Hungary, in the city of Budapest. The company produces not only hormonal contraception, but also other drugs. Particular attention is paid to the following areas of medicine:
- women Health;
- neurology;
- cardiovascular problems;
- over-the-counter drugs.
Dimia contraceptive pills are of high quality, however, they must be taken according to the indications and instructions for use. Gedeon Richter is the choice of people who trust time-tested professional pharmaceuticals.
Pharmacotherapeutic group and properties
The medicine belongs to the group of combined contraceptives aimed at protecting against unwanted pregnancy. This pharmacotherapeutic group includes drugs based on two active ingredients - gestagen and estrogen. The pharmacodynamic and pharmacokinetic properties of the drug are as follows:
- Antimineralocorticoid effect - prevents weight gain and prevents fluid retention in the body.
- Antiandrogenic effect – suppression of the effects of androgens on the body.
- Suppression of ovulation.
- Changes in the properties of cervical secretion.
- Improving the regularity of the menstrual cycle.
The drug "Dimia" is quickly absorbed, regardless of food intake. The substance is eliminated through the kidneys and intestines after 40 hours. When used correctly according to the instructions, the tablets help relieve the painful condition of a woman before her period.
Analogs
The manufacturer of the drug Dimia is Hungarian. Absolute structural analogues of the product, similar in the mechanism of action and chemical composition, are:
- Midiana;
- Angelique;
- Yarina;
- Jess;
- Vidor;
- Dailla;
- Belara;
- Simicia;
- Yarina plus;
- Anabella;
- Delcia;
- Modell trend.
Composition of Dimia
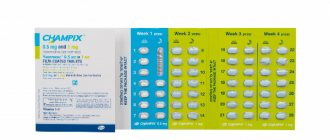
Effective COCs "Dimia" are produced in tablet form. The pack contains 28 tablets, 24 of which contain active substances, and the remaining 4 green tablets are auxiliary. You can often find the name “Dimia” 24 + 4, which indicates the number of active and inactive tablets.
Important! The inactive pills in contraceptives are intended to regulate the menstrual cycle.
In the instructions for use you can find the following information about the composition:
- drospirenone is an active substance that has an antiandrogenic effect;
- ethinyl estradiol - a synthetic analogue of estradiol, promotes thickening of the endometrium;
- lactose monohydrate – used to fill tablets;
- corn starch – holds the components of the drug together;
- macrogol and polyvinyl alcohol copolymer - help the tablet dissolve in water;
- Magnesium stearate – gives tablets their shape.
Each tablet contains 3 mg of drospirenone and 0.02 mg of ethinyl estradiol. Inactive tablets consist of cellulose, starch, magnesium stearate and silicon dioxide.
Price for Dimia tablets
You can purchase the drug dimia at any pharmacy, but you will need to get a prescription from your doctor. You cannot start taking pills on your own or on the recommendation of friends; before starting use, you should definitely visit a specialist. The cost of the drug depends on the region of distribution and the number of tablets in the package; on average, the price for 28 pieces is 700 rubles. The approximate cost of a contraceptive in Moscow is presented in the table:
| Name | Price in rubles |
| Tablets 3 mg+0.02 mg, 28 pcs. | 741 |
| Coated tablets 3mg+0.02mg, tablets, 28 pcs. | 766 |
| Tablets semi-plastic 3mg+0.02mg N28x3 | 1673 |
| Tablets 3 mg+0.02 mg, 84 pcs. | 1732 |
| Dimia tab. p.p.o. 3mg + 0.02mg No. 84 | 1610 |
Indications for use
Only a gynecologist prescribes the use of tablets, regardless of the reason for which Dimia was prescribed. You can start taking pills on your own only after you have fully read the instructions for use.
The main indication for taking a contraceptive will be the ability to protect against unwanted pregnancy. The drug is used as oral contraception - it must be taken orally with water. Doctors often prescribe the drug for medicinal purposes - to correct hormonal levels, eliminate pain before menstruation. You can learn more about the readings from the video
Contraindications to Dimia
Most hormonal contraceptives have contraindications. All prohibitions are indicated in the instructions; if the patient exhibits negative symptoms or develops these conditions after taking the pills, the pills are canceled.
Contraindications to contraceptives "Dimia":
- venous thromboembolism;
- arterial thromboembolism;
- severe liver disease;
- acute or chronic renal failure;
- bleeding from the vagina for unknown reasons;
- the presence of malignant liver tumors;
- the presence of hormone-dependent tumors;
- pregnancy.
The indicated conditions must be present in the anamnesis, then the use of Dimia is prohibited.
Attention! If side effects are observed at the start of treatment, the gynecologist cancels contraception.
Contraindications
The drug has an extensive list of contraindications. It is prohibited to use Dimia if:
- thrombosis or with a predisposition to it;
- migraine;
- diabetes mellitus in combination with vascular disorders;
- liver failure and other pathologies of the internal organ;
- hormonal pathological abnormalities;
- vaginal bleeding.
Contraindications to taking the drug are pregnancy or suspicion of it, the lactation period, an allergic reaction of the body to one or more components of the contraceptive, individual intolerance to peanuts and soy.
Mandatory consultation with a gynecologist before using a contraceptive is necessary if a woman is diagnosed with the following conditions:
- excess weight;
- cardiac pathological conditions;
- hypertension;
- gastrointestinal diseases in the acute phase;
- jaundice;
- herpes;
- tendency to allergic reactions with complications;
- postpartum period.
If, while using a contraceptive, a woman develops one or more of the listed pathological conditions, then taking Dimia should be immediately discontinued.
How to take Dimia
Start taking the product according to the instructions for use with water. The drug must be taken every day at the same time, following the arrows on the blister. The drug is designed for a menstrual cycle of 28 days, so it contains exactly 28 tablets.
How to take Dimia for the first time
If no oral contraceptives have been used in the past month, then the medicine is taken with the first tablet from the first day of the menstrual cycle. The woman chooses a convenient time for her when it is best to take the drug. Now this will need to be done daily.
It is necessary to constantly monitor the intake according to the instructions and not to miss active pills, otherwise you will have to take additional contraceptives. Dimia hormonal tablets begin to act from the first dose, so you can forget about condoms and other methods of protection against unwanted pregnancy.
How to take Dimia green tablets
Tablets colored green do not contain the active substances drospirenone and ethinyl estradiol. On the blister they are located along the arrows, starting with the 25th tablet and ending with the 28th tablet. After the 24th day of the cycle, the reliability of active tablets decreases, so the manufacturer uses a placebo.
Comment! Placebo tablets are necessary to ensure that you do not miss your daily dose of the drug.
Missing 1 green Dimia tablet does not entail anything. If you still missed one inactive tablet, it is recommended not to use it, but simply throw it away. This move will help you avoid confusion when taking the drug further. You often get your period after taking a placebo.
What to do if you missed a Dimia tablet
There are several rules that will help a woman act competently if she missed the Dimia pill. The missed tablet must be taken as soon as the woman remembers it, however, do not forget that the maximum dosage of the drug according to the instructions is no more than two units per day.
Depending on the day of the pass, the following recommendations will apply:
- Days 1-7: the tablet is taken as soon as you remember it. Next, the drug is taken according to the established schedule. In addition, it is necessary to use a barrier method of contraception for the next 7 days.
- Days 8-14: The tablet is taken immediately, even if you need to drink 2 units at the same time. If the medicine was taken according to the regimen a week before the missed dose, then there is no need to take additional protection.
- Days 15-24: Take the tablet immediately, even if you need to take 2 tablets. If the dosage regimen was followed 7 days before the missed date, additional contraception will not be required. If there were the same gaps before, then it is recommended not to take inactive tablets from the last row, but to start a new pack.
Advice! By following the instructions for using Dimia tablets and following the recommended regimen when missed, you can prevent unwanted pregnancy.
How much can you drink Dimia without a break?
In numerous sources you can find information that taking hormonal contraceptives is harmful to a woman’s health with long-term use. The instructions for the drug say the opposite: they can be taken for a long period of time.
You can take the drug without interruption, like other combined hormonal drugs for a long time. Some women take the medicine for 5 years and feel great: the menstrual cycle improves, the pain goes away, there is no need to think about additional contraception. The drug is not suitable for other women, side effects occur, and the doctor discontinues the drug.
When you can not use protection with Dimia
The hormonal drug "Dimia" was created to ensure that sexual partners do not use protection during sex. But the tablets will be effective only in the following cases:
- in full compliance with the instructions for use;
- in the absence of contraindications;
- in the absence of gaps.
If a girl begins to skip taking the drug, there is a high chance of pregnancy: then the use of additional barrier-type contraceptives cannot be avoided. If the patient strictly follows the dosage regimen, you can forget about condoms.
Dimia
Use during pregnancy and breastfeeding
Dimia® is contraindicated during pregnancy.
If pregnancy occurs while using Dimia®, it should be stopped immediately. Extensive epidemiological studies have found neither an increased risk of birth defects in children born to women who took COCs before pregnancy, nor a teratogenic effect of COCs if taken unintentionally during pregnancy.
According to preclinical studies, it is impossible to exclude undesirable effects that affect the course of pregnancy and fetal development due to the hormonal action of the active components.
The drug Dimia® can affect lactation: reduce the amount of milk and change its composition. Small amounts of contraceptive steroids and/or their metabolites may be excreted in milk during COC use. These amounts may affect the child. The use of Dimia® during breastfeeding is contraindicated.
Use for liver dysfunction
Contraindicated:
- existing severe liver disease (or history) provided that liver function is not currently normalized;
- liver tumor (benign or malignant) currently or in history.
Use for renal impairment
Contraindicated:
- severe chronic or acute renal failure
Use in children
The use of the drug before menarche is not indicated.
special instructions
If you have any of the conditions/risk factors listed below, the benefits of taking COCs should be assessed individually for each woman and discussed with her before starting use. If an adverse event worsens or if any of these conditions or risk factors occur, the woman should contact her doctor. The doctor must decide whether to stop taking the COC.
Circulatory disorders
Taking any combined oral contraceptive increases the risk of venous thromboembolism (VTE). The increase in the risk of VTE is most pronounced in the first year of a woman's use of a combined oral contraceptive.
Epidemiological studies have shown that the incidence of VTE in women without risk factors taking low-dose estrogens (<0.05 mg ethinyl estradiol) as part of a combined oral contraceptive is approximately 20 cases per 100,000 woman-years (for “second generation” levonorgestrel-containing COCs) or 40 cases per 100,000 woman-years (for desogestrel/gestodene-containing “third generation” COCs). Women who do not use COCs have an incidence of 5-10 VTE and 60 pregnancies per 100,000 woman-years. VTE is fatal in 1-2% of cases.
Data from a large, prospective, 3-arm study showed that the incidence of VTE in women with or without other risk factors for venous thromboembolism using the combination of ethinyl estradiol and drospirenone 0.03 mg + 3 mg was the same as the incidence of VTE in women using levonorgestrel-containing oral contraceptives and other PDAs. The risk of venous thromboembolism when taking Dimia® has not currently been established.
Epidemiological studies have also revealed an association between COC use and an increased risk of arterial thromboembolism (myocardial infarction, transient ischemic events).
Very rarely, thrombosis of other blood vessels, such as veins and arteries of the liver, mesentery, kidney, brain or retina, has occurred in women taking oral contraceptives. There is no consensus regarding the connection of these phenomena with the use of hormonal contraceptives.
Symptoms of venous or arterial thrombotic/thromboembolic events or acute cerebrovascular accidents:
- unusual unilateral pain and/or swelling of the lower extremities;
- sudden severe pain in the chest, regardless of whether it radiates to the left arm or not;
- sudden shortness of breath;
- sudden onset of cough;
- any unusual severe, long-lasting headache;
- sudden partial or complete loss of vision;
- diplopia;
- impaired speech or aphasia;
- vertigo;
- collapse with or without partial epileptic seizures;
- weakness or very noticeable numbness that suddenly affects one side or part of the body;
- movement disorders;
- symptom complex “acute” abdomen.
Before starting to take COCs, a woman should consult a specialist.
The risk of venous thromboembolic disorders when taking COCs increases with:
- increasing age;
- hereditary predisposition (venous thromboembolism has ever occurred in siblings or parents at a relatively early age);
- prolonged immobilization, extensive surgery, any surgery on the lower extremities or major trauma. In such situations, it is recommended to stop taking the drug (in the case of planned surgery, at least four weeks in advance) and not to resume until two weeks have passed after complete restoration of mobility. If the drug is not stopped promptly, anticoagulant treatment should be considered;
- obesity (BMI more than 30 kg/m2);
- lack of consensus on the possible role of varicose veins and superficial thrombophlebitis in the appearance or exacerbation of venous thrombosis.
The risk of arterial thromboembolic complications or acute cerebrovascular accident when taking COCs increases with:
- increasing age;
- smoking (women over 35 years of age are strongly advised to quit smoking if they want to take COCs);
- dyslipoproteinemia;
- arterial hypertension;
- migraine without focal neurological symptoms;
- obesity (BMI more than 30 kg/m2);
- hereditary predisposition (arterial thromboembolism ever in siblings or parents at a relatively early age). If a hereditary predisposition is possible, a woman should consult a specialist before starting to take COCs;
- damage to the heart valves;
- atrial fibrillation.
Having one major risk factor for venous disease or multiple risk factors for arterial disease may also be a contraindication. Anticoagulant therapy should also be considered. Women taking COCs should be properly instructed to inform their physician if symptoms of thrombosis are suspected. If thrombosis is suspected or confirmed, COC use should be discontinued. It is necessary to start adequate alternative contraception due to the teratogenicity of anticoagulant therapy (indirect anticoagulants - coumarin derivatives).
The increased risk of thromboembolism in the postpartum period should be taken into account.
Other medical conditions associated with adverse vascular events include diabetes mellitus, systemic lupus erythematosus, hemolytic uremic syndrome, chronic inflammatory bowel disease (Crohn's disease or ulcerative colitis), and sickle cell disease.
An increase in the frequency or severity of migraine while taking COCs may be an indication for immediate discontinuation of combined oral contraceptives.
Tumors
The most significant risk factor for developing cervical cancer is infection with the human papillomavirus. Some epidemiological studies have reported an increased risk of cervical cancer with long-term use of combined oral contraceptives, but there remains controversy regarding the extent to which these findings are attributable to confounding factors such as testing for cervical cancer or use of barrier methods of contraception.
A meta-analysis of 54 epidemiological studies found a small increase in the relative risk (RR = 1.24) of breast cancer in women who were currently taking COCs. The risk gradually decreases over 10 years after stopping COC use. Because Breast cancer rarely develops in women under 40 years of age, and an increase in the number of diagnosed cases of breast cancer in COC users has little effect on the overall likelihood of breast cancer. These studies did not find sufficient evidence of causality. The increased risk may result from earlier diagnosis of breast cancer in COC users, the biological effects of COCs, or a combination of both factors. Diagnosed breast cancer in women who had ever taken COCs was clinically less severe, which was due to early diagnosis of the disease.
Rarely, benign liver tumors and, even more rarely, malignant liver tumors have occurred in women taking COCs. In some cases, these tumors were life-threatening due to intra-abdominal bleeding. This should be taken into account when making a differential diagnosis in the event of severe abdominal pain, liver enlargement, or signs of intra-abdominal bleeding.
Other states
The progestogen component of the drug Dimia® is an aldosterone antagonist that retains potassium in the body. In most cases, an increase in potassium levels is not expected. However, in a clinical study in some patients with mild to moderate kidney disease who were taking potassium-sparing medications, serum potassium levels increased slightly while taking drospirenone. Therefore, it is recommended to monitor serum potassium levels during the first cycle of treatment in patients with renal failure whose pre-treatment serum potassium concentrations were at the upper limit of normal and, especially, while taking potassium-sparing drugs.
Women with hypertriglyceridemia or a hereditary predisposition to it may have an increased risk of pancreatitis when taking COCs.
Although slight increases in blood pressure were observed in many women taking COCs, clinically significant increases were rare. Only in these rare cases is it justified to immediately stop taking the COC. If, when taking COCs in patients with concomitant arterial hypertension, blood pressure constantly increases or significantly elevated blood pressure cannot be corrected with antihypertensive drugs, taking COCs should be discontinued. After normalization of blood pressure with the help of antihypertensive drugs, COC use can be resumed.
The following diseases appeared or worsened both during pregnancy and when taking COCs, but the evidence of their relationship with taking COCs is inconclusive: jaundice and/or itching associated with cholestasis, gallstones; porphyria; systemic lupus erythematosus; hemolytic-uremic syndrome; rheumatic chorea (Sydenham's chorea); herpes during pregnancy; otosclerosis with hearing loss.
In women with hereditary angioedema, exogenous estrogens may induce or worsen symptoms of edema.
Acute or chronic liver disease may be an indication to stop taking COCs until liver function tests normalize. Recurrence of cholestatic jaundice and/or pruritus associated with cholestasis, which developed during a previous pregnancy or with earlier use of sex hormones, is an indication for discontinuation of COCs.
Although COCs may affect peripheral insulin resistance and glucose tolerance, changes in treatment regimen in patients with diabetes mellitus while taking low-hormone COCs (containing <0.05 mg ethinyl estradiol) are not indicated. However, women with diabetes should be closely monitored, especially in the early stages of taking COCs.
While taking COCs, an increase in endogenous depression, epilepsy, Crohn's disease and ulcerative colitis was observed.
Chloasma may occur from time to time, especially in women who have a history of chloasma during pregnancy. Women with a tendency to chloasma should avoid exposure to the sun or ultraviolet light while taking COCs.
Drospirenone + ethinyl estradiol coated tablets contain 48.53 mg of lactose monohydrate, placebo tablets contain 37.26 mg of anhydrous lactose per tablet. Patients with rare hereditary diseases such as galactose intolerance, lactase deficiency or glucose-galactose malabsorption who are on a lactose-free diet should not take this drug.
Women who are allergic to soy lecithin may experience allergic reactions.
The effectiveness and safety of Dimia® as a contraceptive have been studied in women of reproductive age. It is assumed that in the postpubertal period up to 18 years of age, the effectiveness and safety of the drug are similar to those in women after 18 years of age. The use of the drug before menarche is not indicated.
Medical examinations
Before starting or re-using Dimia®, obtain a complete medical history (including family history) and exclude pregnancy. It is necessary to measure blood pressure and conduct a medical examination, guided by contraindications and precautions. A woman should be reminded to carefully read the instructions for use and adhere to the recommendations contained therein. The frequency and content of the survey should be based on existing practice guidelines. The frequency of medical examinations is individual for each woman, but should be carried out at least once every 6 months.
Women should be reminded that oral contraceptives do not protect against HIV infection (AIDS) and other sexually transmitted diseases.
Reduced efficiency
The effectiveness of the COC may be reduced, for example, if you skip a dose of drospirenone + ethinyl estradiol tablets, have gastrointestinal disorders while taking drospirenone + ethinyl estradiol tablets, or take other medications at the same time.
Insufficient cycle control
As with other COCs, a woman may experience acyclic bleeding (spotting or withdrawal bleeding), or if two bleedings are missed, pregnancy should be ruled out before continuing to take the COC.
Impact on the ability to drive vehicles and operate machinery
Not found.
Pregnancy after Dimia
Conception after taking it occurs during the period of drug withdrawal. As a rule, the lack of protection makes itself felt: unprotected sexual intercourse threatens an early pregnancy. However, doctors do not recommend rushing with fertilization, because the body is in a stage of hormonal stress after stopping oral contraception.
According to reviews from girls, pregnancy after discontinuation occurred in the 2nd month, although doctors recommend waiting at least 3-4 months. This is necessary so that the female body can rest and recover after taking hormones. By allowing the body to adjust to its previous hormonal levels, a woman contributes to natural preparation for conception.
Side effects of Dimia
The manufacturer indicates in the instructions that side effects may occur during use. The likelihood of their occurrence is small, but still a small percentage exists:
- Gastrointestinal tract: weight gain, anorexia, nausea, diarrhea, increased appetite.
- Allergic reactions from the immune system.
- Nervous system: changeable mood, headaches, dizziness, nervousness, insomnia, rarely – lack of orgasm.
- Vascular: migraine, varicose veins, rarely – fainting.
- Reproductive system: chest pain, lack of menstruation after withdrawal, pelvic pain, painful menstruation.
According to statistics, some women experienced venous and arterial thromboembolic diseases.
Is it possible to gain weight from Dimia?
It is necessary to purchase and use hormonal contraceptives only as prescribed by a doctor and according to the instructions for use. This helps avoid unwanted side effects such as weight gain. According to statistics, hormonal pills "Dimia" in rare cases can cause weight gain.
Women note a slight increase in appetite, which contributes to a weight gain of 2-3 kg. However, not every girl experiences this side effect. Some patients do not notice that weight gain occurred for another reason, however, they associate this phenomenon with taking the drug. According to reviews, the weight gained in the first month after taking it was subsequently successfully eliminated.
Chest hurts from Dimia
Another most common side effect is breast pain. This phenomenon occurs for the following reasons:
- breast enlargement;
- approaching menstruation;
- presence of breast diseases.
If all the tests performed have shown that the listed conditions are absent and the chest hurts precisely from birth control pills, the doctor should cancel it and prescribe the patient another drug. Chest pain may be associated with hormonal changes and adaptation, then they disappear the next month and the woman continues treatment.
Side effects
According to reviews, Dimia can cause the following unwanted side effects:
- Lymphatic system and hematopoietic system: thrombocytopenia, anemia.
- Metabolism: anorexia, weight loss or gain, increased appetite, hyponatremia, hyperkalemia.
- Infections: candidiasis.
- Immune system: development of various allergic reactions.
- Nervous system: dizziness, headache, paresthesia, tremor, vertigo, depression, insomnia, anorgasmia, drowsiness, decreased libido.
- Cardiovascular system: varicose veins, migraine, nosebleeds, tachycardia, fainting, increased blood pressure.
- Digestive system: diarrhea, vomiting, nausea, abdominal pain.
- Musculoskeletal system: pain in the limbs and back, muscle cramps.
- From the skin and subcutaneous tissue: itching, rash, eczema, dry skin, skin stretch marks, contact dermatitis.
- Biliary tract and liver: cholecystitis, gallbladder pain.
- Reproductive system: breast pain, absence of bleeding, enlarged mammary glands, pelvic pain, vaginitis, vaginal discharge, heavy or scanty bleeding, dry vaginal mucosa, cervical polyps, cyst or breast hyperplasia.
Special instructions and precautions
It is necessary to take contraception only according to the instructions, which also indicate special cases when caution should be exercised:
- smoking women under 35 years of age;
- tendency to obesity;
- arterial hypertension;
- migraine;
- heart defects;
- predisposition to thrombosis.
All of these conditions can lead to thromboembolism, which significantly worsens the patient’s condition when taking the medication. In addition, those women who have developed various diseases after taking other COCs should take the drug with caution.
Drug interactions
Many girls are interested in the issue of interactions with other medications. The manufacturer provides the following information in the instructions for use:
- Primidone, barbiturates and phenytoin weaken the contraceptive effect. This also includes preparations containing St. John's wort.
- Substances that increase or decrease the concentration of estrogen - inhibitors of HIV or hepatitis C virus proteases.
- Drugs containing itraconazole, clarithromycin, erythromycin, and grapefruit juice can increase the concentration of estrogen in the blood plasma.
Important! While taking medications that reduce the effect, you should consult your doctor for possible additional prescription of other contraceptives.
Dimia and alcohol
The compatibility of Dimia and alcohol should not raise doubts, because both substances are absorbed and processed separately. However, with simultaneous use, there is a heavy load on the liver, where processing occurs. You should not take Dimia and alcohol for the following reasons:
- increased side effects may occur;
- The effectiveness of the drug may decrease.
If it is impossible to give up alcohol in certain situations, then it is recommended to take alcohol 3 hours after the pill.
Dimia and smoking
As mentioned above, smoking is not advisable for women under 35 years of age while taking contraceptives. This combination not only harms the woman’s general health, but also increases the risk of thrombosis. Women who have a history of problems with blood vessels need to be extremely careful, because smoking impedes the movement of blood through the veins. All listed risks can be read in the instructions for use.
Reviews
I have been taking Dimiya for 4 months now and I have no problems. I get only advantages from this treatment - during this time I was able to lose 5 kg. Now I almost never get acne, my skin is no longer oily. Before that, I actively used various masks and tonics, but when I started taking Dimia tablets, I forgot about it. Anna
I recently started treatment with Dimia.
Before that, I took other pills, but they greatly interfered with my libido, but Dimia does not give the same effect. With it, my skin became more matte, the shine disappeared, the impressions were only the most positive. My husband is also happy about my flourishing state, and the reason for this is that my PMS has finally disappeared and I behave much calmer. Ilona
After I switched to the second cycle, my acne disappeared. But the effect of the drug did not end there. After some more time, my skin finally healed and tightened. I am glad that the skin is no longer greasy, and this is a guarantee that new rashes will not appear. Vika
Terms and conditions of storage
Dimia tablets, photos of which can be seen in this material, are sold in pharmacies with a prescription. Storage recommendations will be as follows:
- storage is carried out for no more than 2 years;
- the drug must be stored in a dark place;
- It is not allowed to store the contraceptive in a place accessible to children.
After the expiration date, the medicine cannot be used, as indicated in the instructions for use. Expiration dates can be seen on the package of the drug, as well as on the blister of tablets.
Dimia price in pharmacies
The cost of Dimia will vary depending on the place of sale: you can buy the drug in city pharmacies and online. Hormonal pills are sold in small and large packs, designed for several months of use:
- 28 pieces in a pack - from 640 to 720 rubles.
- 84 pieces in a pack - from 1600 to 1800 rubles.
It is recommended to purchase a large pack if the woman has gone through the adaptation period without side effects. For medicinal purposes, a doctor may prescribe the drug for six months, so a large pack is designed for half of this period.
How often should you visit a doctor?
While taking the drug Dimia and its analogues, a woman must be regularly monitored by a gynecologist. to undergo examination by this specialist at least once every 6 months
During it, the woman will have to undergo, along with a visual examination, cytological smears, colposcopy, palpation of the mammary glands, and blood pressure measurement. In special cases, special examinations may be prescribed - ultrasound, biochemical blood tests, etc.
Dimia's analogs
Substitutes for the drug should be based on a similar active ingredient - ethinyl estradiol. Such drugs include:
- "Yarina";
- "Belar";
- "Silhouette";
- "Lindynet 20".
Dimia or Belara: which is better
The drug "Belara" contains ethinyl estradiol and chlormadinone. If the first component is a synthetic analogue of the female hormone, then chlormadinone in the drug is responsible for the antiandrogenic effect. Contraindications to the drug are the same as for Dimia - they are indicated in the instructions for use.
Gynecologists can transfer a patient from Dimia to Belara if individual side effects occur. "Belara" has the same cost as "Dimia".
Dimia or Silhouette: which is better
The composition of the drug "Silhouette" contains a combination of the substances ethinyl estradiol and dienogest - a gestagen close in origin to progesterone. "Silhouette" is prescribed not only for oral contraception, but also for the treatment of acne associated with hormones.
“Silhouette” will be similar to “Dimia”, only cheaper. It can be purchased from 600 rubles per pack of 21 pieces. There is a slightly different method of administration here: when all the tablets are taken, a seven-day break is taken, after which they begin to take the next pack. According to the instructions for use, your period comes during this break.
Dimia or Lindinet 20: which is better?
"Lindinet 20" in addition to ethinyl estradiol, contains gestodene - a progestogen. There are no auxiliary inactive tablets; each tablet contains an active ingredient: more details can be found in the instructions for use. Lindinet 20, like Dimia, is prescribed for oral contraception.
Lindinet 20 has a lower cost than Dimia, so gynecologists often prescribe it to women who have decided to use oral contraception for the first time. Over time, the doctor may recommend switching to Dimia.
Dimia or Yarina: which is better
COCs "Yarina" are one of the most common drugs used not only for contraception, but for medicinal purposes. "Yarina" is prescribed for:
- normalization of the menstrual cycle;
- treatment of endometriosis;
- treatment of cysts;
- eliminating pain in the first days of menstruation.
Yarina also contains drospirenone and ethinyl estradiol. The drugs are equally well tolerated by patients, so they are prescribed interchangeably.
pharmachologic effect
The combination drug Dimia is a monophasic oral drug. This medicine contains ethinyl estradiol and drospirenone (a naturally occurring progesterone analogue). The active substances that make up the drug do not have estrogenic, antiglucocorticoid, or glucocorticoid properties. The medicine achieves its effectiveness due to changes in the endometrium, inhibition of ovulation and an increase in the viscosity of the cervical secretion, which prevents sperm from penetrating into its cavity.
After taking the drug orally, the active substances are completely absorbed into the bloodstream from the small intestine. They are distributed evenly throughout all tissues of the body. The maximum concentration of the drug is reached two hours after administration. The breakdown products of ethinyl estradiol and drospirenone are excreted from the body primarily in the urine.
Reviews from women about Dimia
To understand the effect of the drug "Dimia" on the female body, it is not enough to read the instructions for use. It would be a good idea to study the reviews of girls who took the pills for a certain time.
Reviews of women about Dimia after 30 years
Elizaveta Andreevna Kruglova, 32 years old, Pskov
I am a mother of two children, in order to prevent a third pregnancy, I went to the gynecologist to be prescribed hormonal contraception. The doctor offered a choice of “Jess”, “Dimia” and “Lindinet”. After reading the girls’ reviews about “Dimia”, I decided to choose it - it’s a kind of analogue of “Jess”, only more accessible. I have been taking Dimia for 3 months now and have noticed stable periods that come at the same time. Libido also increased, acne on the face disappeared. I recommend the drug, it worked for me.
Palagina Tatyana Yurievna, 38 years old, Nizhny Novgorod
When my doctor prescribed me hormonal pills "Dimia", I was shocked, I was afraid to take them because of the huge number of side effects. At home I read reviews about weight gain from Dimia, the girls wrote that they had gained a little weight. I started taking it at my own risk and did not notice any weight gain even during the adaptation period. The tablets perfectly protect against pregnancy, they are convenient to take, there is a diagram on the pack, and the instructions for use contain a complete list of side effects.
Reviews of women about Dimia after 40 years
Mukhina Ekaterina Viktorovna, 45 years old, Yaroslavl
I had previously taken other Jess birth control pills, but that was 10 years ago. Recently I visited a gynecologist, she advised me to pay attention to Dimia, especially since it is a generic version of Jess. I bought it, read the instructions for use, and it’s true – the composition is similar. I've been taking it for the second month and there are no side effects. On the contrary, the condition of my skin has improved, and I can more easily endure the onset of menopause.
Kovalchuk Anna Semenovna, 43 years old, Ivanovo
The gynecologist prescribed Dimia as oral contraception and to normalize the menstrual cycle. I read the instructions for use and started drinking. I am satisfied with the drug, since not every pill after 40 years is suitable for women. During the adaptation period, my chest hurt a little, but in the second month there were no side effects.
Interaction of Dimia with other drugs
The effectiveness of the contraceptive can be weakened by combined use of the drug with barbiturates (a group of drugs derived from barbituric acid) and drugs that affect liver enzymes: Griseofulvin, Oxcarbazepine, Topiramate, Phenytoin, Primidone, Felbamate, Rifampicin. In addition, the instructions indicate that drugs that contain St. John's wort in their chemical composition, when used simultaneously with dimia, induce (stimulate) microsomal liver enzymes, which also negatively affects the female body.
A decrease in the circulation of estrogen and at the same time the effectiveness of the contraceptive occurs when Ampicillin and Tetracycline are used simultaneously with antibiotics. HIV protease inhibitors and their combinations have a negative effect on the hepatic metabolism of the drug. Women undergoing short-term treatment with any of the above drugs should temporarily use barrier methods of contraception (condom).