Quick transition Treatment of primary immunodeficiency
Primary immunodeficiency (PID) is a heterogeneous group of rare, predominantly hereditary diseases characterized by disruption of one or more components of the immune system.
Such immunity disorders lead to increased vulnerability of the body to various pathogens, resulting in frequent and recurrent infectious diseases, the development of chronic and systemic diseases (autoimmune, cancer).
Classification of immunodeficiency states
Based on their origin, all immunodeficiency states are divided into three main groups:
1. Physiological.
2. Primary immunodeficiency states. May be congenital or hereditary. Primary immunodeficiency states arise as a result of a genetic defect, under the influence of which the functioning of the cells of the immune system of the human body is disrupted.
3. Secondary immunodeficiency states (acquired after birth, during life). They develop under the influence of various biological and physical factors.
Based on the primary damage to the cells of the body’s immune system, immunodeficiency states are divided into 4 groups:
- with damage to humoral immunity (humoral, B-dependent);
- with damage to cellular immunity (cellular, T-dependent);
- with damage to phagocytosis (A-dependent);
- combined (when both humoral and cellular immunity are affected).
Secondary immunodeficiency
Secondary immunodeficiencies occur in animals in the postnatal period under the influence of numerous immunosuppressants.
Immunosuppressive diseases are often characterized by a violation of the genesis and functions of immunocytes and nonspecific protective factors. The relationship between the state of the immune system and the pathogenic agent is very complex. Secondary immunodeficiencies can be the result of poor nutrition of animals, infections and infestations, unfavorable living conditions, exposure to chemical and cytotoxic substances, physical factors, metabolic diseases and many other reasons.
Bacterial and viral infections can be both a consequence and a cause of secondary immunodeficiencies.
In acute infectious diseases (viral hepatitis, paratyphoid fever, plague, parainfluenza, etc.) in both humans and animals, immunodeficiency states have common patterns: in the vast majority of patients, the T-immune system primarily suffers with a decrease in the reproduction of T-active cells - helpers, impaired differentiation of populations, decreased delayed-type hypersensitivity.
The B-system of immunity is affected to a lesser extent.
It was revealed that when two or more infectious diseases are combined, immunodeficiency is more severe than with a monoinfection. With the development of immunodeficiency against the background of an existing congenital or acquired immunodeficiency, the indicators of immunity and nonspecific protective factors are reduced to a minimum, and the disease becomes severe and often fatal.
Researchers of viral infections of farm animals show particular interest in the state of body resistance and secondary immunodeficiency.
Thus, the fact of pressure on the cellular link of immunity in parainfluenza, frost plague, infectious anemia of horses, canine distemper, and Marek's disease in birds has been established. In Aujeszky's disease, the migration of macrophages is inhibited, delayed-type hypersensitivity is weakened, and the T-system of immunity is affected.
African swine fever is characterized by increased blast transformation, inhibition of leukocyte migration, and enhanced antibody-dependent cytolysis; with viral gastroenteritis of calves, antibodyogenesis is suppressed; with transmissible gastroenteritis of pigs, the proliferation of leukocytes is inhibited.
It has been revealed that many viruses that cause infectious diseases have tropism for lymphoid cells, primarily macrophages, which ensures their reproduction and dissemination. The interaction of viruses with lymphoid tissue plays a central role in the pathogenesis of viral diseases with systemic pathology.
Thus, the development of a viral infection of various types or even a post-vaccination process in an animal body can significantly change its immune status and determine virus-induced immunodeficiency.
Immunodeficiency during helminthic and protozoal infestations has been studied in experimental models and in animals of different species with spontaneously occurring diseases.
Experiments have shown that the host organism infected by the parasite gives a reduced immune response to heterologous antigens. The immunosuppressive effect was established when experimental animals were infected with protozoa (Babesia microti, Toxoplasma gondii, Trypanasoma brucei) and helminths (Fasciola hepatica, Trichinella spiralis, Schistosoma mansoni).
In infected animals, antibody production was suppressed and cellular immunity was inhibited, which was assessed by rejection of skin allografts, response to skin-sensitizing agents and to T-cell mitogens.
The mechanisms of immunodeficiency of parasitic etiology are far from fully understood. It is believed that numerous factors that occur in host-parasite systems are involved. Thus, in the pathogenesis of developing secondary immunodeficiency during parasitosis, nonspecific suppressor cells found in the spleen of mice infested with trypanosomes are important.
These cells are capable of suppressing the response of normal spleen cells to both T and B cell mitogens. The functions of macrophages in animals with experimental malaria are significantly impaired. They are not able to fully present antigens.
Malaria and trypanosomiasis are accompanied by a sharp increase in the level of immunoglobulins, from which the conclusion is drawn about polyclonal activators of lymphocytes of a parasitic nature. Long-term polyclonal activation of B cells leads to a progressive decrease in antigen-sensitive B cells and a weakening of the humoral response to heterologous antigens.
Excretory and secretory products of the liver fluke have a cytotoxic effect on lymphocytes. The blood serum of animals infected with the nematode Trichinella spiralis is capable of agglutinating and then killing lymph node cells. Tryptofol, synthesized by Tripanosoma gambiense, can inhibit the production of antibodies in experimental animals.
Some parasites inhibit the immune response by altering the physiological functions of the host. Thus, they can enhance the production of corticosteroid hormones.
Experiments have established that the administration of steroid drugs promotes more intense helminth infection. There have even been cases of death after infection of animals with Strongyloides stercoralis that received cortisone.
In case of naturally occurring and artificially caused Fasciola hepatica invasion of cattle, with hastilesiosis and gastrointestinal strongylatosis of sheep, and trichinosis, suppression of the T-cell immune response was noted.
Infestation of sheep with Strongyloides papiloides leads to a decrease in weight and cellular-lymphoid organs, and a sharp activation of T-suppressors.
Thus, the immunological deficiency caused by parasitic invasion is based on a variety of mechanisms. These are excessive activity of suppressor cells, the presence of common antigenic determinants in the parasite and host, dysfunction of macrophages, polyclonal activation of B cells, parasitic factors that inhibit lymphocytosis.
To identify a complete picture of the inhibitory effect of pathogens of parasitic diseases, much remains to be discovered. However, the increased susceptibility of infested animals to infections caused by other pathogens is already obvious. Vaccination against heterologous infectious agents may be less effective in them. They are more prone to developing “spontaneous” tumors and tumors caused by viruses and carcinogenic agents. The host's orgasm loses the ability to effectively defend itself against the homologous parasite.
This tolerance determines the chronic course of many invasive diseases.
The induced form of VIN occurs as a result of exposure to specific causative factors: X-ray radiation, cytostatic therapy, the use of glucocorticoids, trauma and surgical interventions.
This group also includes immune disorders that develop secondary to the underlying disease (diabetes, liver disease, kidney disease, malignant neoplasms).
Clinical signs suggesting the presence of VIN:
• chronic, frequently recurring bronchitis with a history of pneumonia, combined with diseases of the ENT organs (purulent sinusitis, otitis, lymphadenitis);
• frequently recurring pneumonia, bronchopleuropneumonia;
• bronchiectasis;
• bacterial lesions of the skin and subcutaneous tissue (pyoderma, furunculosis, abscesses, phlegmon, septic granulomas, recurrent paraproctitis in adults);
• fungal infections of the skin and mucous membranes, candidiasis, parasitic diseases;
• aphthous stomatitis in combination with an increased incidence of acute respiratory viral infections;
• recurrent herpesvirus infection of various localizations;
• gastroenteropathy with chronic diarrhea of unknown etiology, dysbacteriosis;
• lymphadenopathy, repeated lymphadenitis;
• prolonged low-grade fever, fever of unknown etiology;
• generalized infections (sepsis, purulent meningitis).
Causes of immunodeficiency conditions
Since there are quite a lot of reasons for the violation of the body’s immune defense, they are conditionally divided into several groups.
The first group includes congenital immunodeficiency conditions, when the disease manifests itself immediately after birth or in early childhood.
The second group includes secondary immunodeficiency conditions, the cause of which may be:
- cachexia;
- rubella, herpes, measles viruses;
- prolonged fasting;
- blood poisoning;
- poisoning by chemicals or drugs;
- tuberculosis;
- autoimmune diseases;
- the presence of parasites in the patient’s body;
- complications after surgery;
- HIV;
- old age, pregnancy;
- oncological diseases;
- metabolic disorder;
- diseases of endocrine organs;
- large blood loss;
- chronic stress.
Treatment with folk remedies: is it a myth?
Yes, a myth. Folk remedies for the treatment of a dangerous syndrome do not bring the desired effect, and stimulation of healthy immunity “for prevention” is called into question. Conducted studies show that the advertised echinacea for the body only works as a placebo. It is not recommended by doctors to be seduced by dietary supplements on pharmacy shelves to increase immune defense. Medicines are vital for patients who carry the human immunodeficiency virus - treatment without them is impossible, but they are more likely to harm a healthy body.
To combat harmful environmental factors, what is required is not “bulletproof armor”, but a system capable of adapting and self-renewing, acquiring new skills. If you suspect immunodeficiency or HIV, treatment with folk remedies should not be carried out; you should immediately consult a doctor.
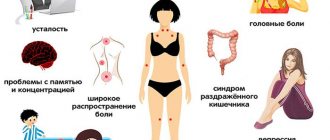
Symptoms of immunodeficiency conditions
Both acquired and congenital immunodeficiency conditions have similar clinical signs:
- increased susceptibility to any infections;
- aches and pain in muscles, bones, joints;
- frequent exacerbations of chronic diseases (arthrosis, arthritis, tonsillitis, respiratory diseases, gastrointestinal tract, and so on);
- painful and enlarged lymph nodes;
- a combination in one disease of several pathologies of different etiologies (fungal, bacterial, viral);
- constantly elevated body temperature (up to 37.7 degrees);
- low effectiveness of taking antibiotics;
- general weakness, causeless fatigue, lethargy, depressed mood;
- increased sweating.
In addition to the clinical picture, when making a diagnosis, it is necessary to identify the cause of immunodeficiency. This is necessary in order to develop the correct treatment regimen and not cause even more harm to the body.
Immunodeficiency conditions in children
IDS in children develop as a result of damage to one or several parts of the body’s immune defense.
Immunodeficiency states manifest themselves in children in the form of severe and often recurrent infections. It is also possible to develop tumors and autoimmune manifestations against the background of immunodeficiency.
Some types of IDS in children manifest themselves, including in the form of allergies.
There are two types of immunodeficiency in children: primary and secondary. Primary immunodeficiencies are caused by genetic changes and are less common than secondary immunodeficiencies, which are caused by external influences or a disease.
AIDS (acquired immune deficiency syndrome)
With the help of gp120 (surface glycoprotein), HIV attaches to the antigen-CD4 receptor and co-receptor located on the surface membrane of cells. For T lymphocytes the co-receptor is CXCR-4, and for macrophages it is CCR-5. The cell membranes of the cell and the virus fuse, the virus enters the cell, where the viral RNA is released from the capsid and begins, using reverse transcriptase, to copy two strands of DNA onto the viral RNA (reverse transcription).
The produced DNA penetrates into the nucleus of the host cell and is integrated into the host chromosome with the help of the enzyme integrase. With the help of RNA polymerase, the synthesis of the viral genome and messenger RNA of viral proteins begins. Viral enzymes and structural proteins are read from messenger RNA on the cell's ribosomes.
The synthesized RNAs leave the cell nucleus into the cytoplasm, where the formation of a new virus begins. The viral genome is ordered by the enzyme protease, and with the help of gp41 and gp120 a new viral envelope is formed. New viral particles bud from the surface of the cell, capturing part of its membrane, and the viruses enter the bloodstream, and the host CD4+ lymphocyte dies.
During the acute phase of HIV infection, the absence of a specific immune response allows the virus to actively replicate and reach high concentrations in the blood. The virus colonizes various tissues, primarily the organs of the lymphatic system, and destroys CD4 lymphocytes.
In addition to CD4 lymphocytes (helpers), CD8 lymphocytes and macrophages, the virus can infect other cells: alveolar macrophages of the lungs, Langerhans cells, follicular dendritic cells of lymph nodes, oligodendroglial cells and astrocytes of the brain, epithelial cells of the intestine.
In lymphoid tissue, HIV multiplies throughout HIV infection, affecting macrophages, activated and resting CD4 lymphocytes, and follicular dendritic cells. The number of cells containing proviral DNA in lymphoid tissue is 5-10 times higher than among blood cells, and HIV replication in lymphoid tissue is 1-2 times higher than in blood. Thus, the main reservoir of HIV is the lymph nodes.
In addition, the virus persists in the dendritic cells of the lymph nodes for a long time after a period of acute viremia and also serves as a reservoir of infection.
Activation of CD8 lymphocytes and the formation of antigen-specific cytotoxic T lymphocytes requires the presentation of a peptide antigen in combination with a class I human leukocyte antigen (Human leukocyte antigen). Dendritic cells are required to initiate primary antigen-specific responses. They capture antigens, process them and transfer them to their surface, where, in combination with additional stimulating molecules, they activate T lymphocytes. Infected cells often do not release additional stimulatory molecules and are therefore unable to produce sufficient numbers of response cells (B and T lymphocytes), which depend on dendritic cells for function.
After reverse transcription is completed in the CD4 lymphocyte, the viral genome is represented by proviral unintegrated DNA. Activation of T lymphocytes is required for the integration of proviral DNA into the genome of the host cell and the formation of new viruses. The contact of CD4 lymphocytes and antigen-presenting cells in lymphoid tissue, the presence of viruses on the surface of follicular dendritic cells and the presence of proinflammatory cytokines (IL-1, IL-6 and TNF-α) promote and support the replication of HIV in infected cells. Therefore, lymphoid tissue serves as the most favorable environment for HIV replication.
Genetic factors Some genetic factors may protect against HIV infection. For example: People with mutations in CCR5 (coreceptor for M-tropic strains of the virus) are little or not at all susceptible to M-tropic strains of HIV-1, but are infected with T-tropic strains.
Homozygosity for HLA-Bw4 is a protective factor against disease progression. In heterozygotes for HLA class I loci, immunodeficiency develops more slowly than in homozygotes.
Studies have shown that in carriers of HLA-B14, B27, B51, B57 and C8 the infection progresses more slowly, while in carriers of HLA-A23, B37 and B49 immunodeficiency develops quickly. All HIV-infected people with HLA-B35 developed AIDS no earlier than 8 years after infection.
Studies have also shown that sexual partners who are HLA class I incompatible have a lower risk of contracting HIV through heterosexual intercourse.
Immunity in AIDS In the acute phase of HIV infection, in the stage of viremia, there is a sharp decrease in CD4+ T-lymphocytes due to the direct lysing effect of the virus and an increase in the number of copies of viral RNA in the blood.
After this, there is a stabilization of the process with a slight increase in the number of CD4 cells, which, however, does not reach normal values.
The positive dynamics are due to an increase in the number of cytotoxic CD8+ T-lymphocytes. These lymphocytes are capable of killing HIV-infected cells directly by cytolysis without restriction to class I human leukocyte antigen (HLA).
In addition, they secrete inhibitory factors (chemokines), such as RANTES, MIP-1alpha, MIP-1beta, MDC, which prevent virus replication by blocking coreceptors.
HIV-specific CD8+ lymphocytes play a major role in the control of the acute phase of HIV infection, but in the chronic course of infection it does not correlate with viremia, since: • The proliferation and activation of CD8+ lymphocytes depends on antigen-specific CD4 T helper cells. • CD8+ lymphocytes can also become infected with HIV, which can lead to a decrease in their numbers.
Acquired immunodeficiency syndrome is the terminal stage of HIV infection; it develops in most patients when the number of CD4+ T-lymphocytes and blood drops below 200 cells/ml (the norm for CD4+ T-lymphocytes is 1200 cells/ml).
The depression of CD4+ cells during HIV infection is explained by the following theories: • Death of CD4+ T lymphocytes as a result of the direct cytopathic effect of HIV • HIV primarily affects activated CD4 lymphocytes, and since HIV-specific lymphocytes are among the first cells activated during HIV infection infections, they are among the first to suffer. • Changes by the virus in the cell membrane of CD4+ T-lymphocytes, which leads them to merge with each other with the formation of giant syncytia, which is regulated by LFA-1 (Lymphocyte function-associated antigen 1) • Catastrophe of CD4 cells by antibodies, as a result of antibody-dependent cytotoxic action (eng. ADCC-antibody-dependent cellular cytotoxicity). • Activation of natural killer cells[49][50]. • Autoimmune catastrophe • Binding of the viral protein gp120 to the CD4 receptor (CD4 receptor masking) and, as a result, the inability to recognize the antigen, the inability of CD4 to interact with HLA class II. • Programmed cell death. • Anergy of the immune response (Greek Ανεργία - idleness, idleness, English Anergy).
B-lymphocytes during HIV infection undergo polyclonal activation and secrete large amounts of immunoglobulins, TNF-α, interleukin-6 and the DC-SIGN lectin, which promotes the penetration of HIV into T-lymphocytes.
In addition, there is a significant decrease in interleukin-2, produced by type 1 CD4 helper cells and which is critical in the activation of cytotoxic T-lymphocytes (CD8+, CTL) and suppression by the virus of the secretion of interleukin-12 by macrophages, a key cytokine in the formation and activation of type 1 T-helper cells and NK lymphocytes (natural killer cells).
Antibodies to HIV According to some data, in 99% of those infected, antibodies are detected within the first 12 weeks (6 - 12 weeks) after initial contact with the virus. According to other data: in 90-95% within 3 months after infection, in 5-9% - after 6 months, 0.5-1% - at a later date).
The period with a false negative antibody result is called the “window period,” during which the infected person may already be the source of the infection. The first antibodies detected are the “gag” (English group antigen) HIV proteins p24 and p17, as well as the p55 precursor. The formation of anti-p24 antibodies is combined with a decrease in the levels of free p24 antigen detected in the blood before the appearance of antibodies.
Following anti-p24 antibodies, antibodies appear against the Env (Envelope) proteins - gp160, gp120, p88, gp41 and the pol (Polymerase) gene - p31, p51, p66.
Antibodies against the genes “vpr”, “vpu”, “vif”, “rev”, “tat”, “nef” can also be detected [69][70].
The most studied antibodies are those directed against “Env” proteins - gp120, gp41. They are divided into two classes: type-specific and group-specific.
Another group of anti-gp120 antibodies, involved in antibody-dependent cytotoxicity (ADCC) and the destruction of HIV-infected CD4+ cells, can also destroy uninfected cells whose receptors are bound by free gp120 circulating in the blood - an effect called: Innocent bystanders (Bystander killing ).
Diagnosis of immunodeficiency conditions
When making a diagnosis, the doctor first of all pays attention to the family history. He finds out whether there are cases of autoimmune diseases, early death, cancer at a relatively young age in the family, and so on. Another sign of IDS may be an adverse reaction to vaccination.
After the interview, the doctor begins the examination. It is necessary to pay attention to the patient's appearance. As a rule, a person suffering from immunodeficiency appears sickly. He has pale skin, which often has various types of rashes. A person complains of constant weakness and fatigue.
In addition, he may have inflamed eyes, chronic diseases of the ENT organs, a constant cough, and swelling of the nostrils.
To clarify the diagnosis, additional examination is carried out, which may include:
- blood test (general, biochemical);
- Analysis of urine;
- screening tests;
- determining the level of immunoglobulins in the blood and so on.
If it turns out that the patient has a recurrent infection, then measures are taken to eliminate it. If necessary, smears can be taken and then examined under a microscope to identify the causative agent of infection.
What diagnostics should I undergo?
If immunodeficiency is suspected, contact an immunologist. A history is taken and a general examination is carried out to detect visible pathologies and abnormalities. The specialist prescribes the main diagnostic procedure - a blood test. Two levels of assessment of the biomaterial, in each of which the number of certain cells is counted, and the response to external stimuli is determined, indicating the presence or absence of malfunctions in the immune system. Additional studies are necessary to establish an accurate diagnosis:
- skin tests - checking for candida infection (for mucocutaneous immunodeficiency);
- fluoroscopy - examination of the position of the ribs (pathologies are characteristic of a deficiency of certain enzymes);
- cardiogram - abnormalities of the heart indicate DiGeorge syndrome.
Genetic testing is also used to identify defects in DNA.
Treatment of immunodeficiency conditions
Immunodeficiency conditions are treated with antibacterial, antiviral, antifungal drugs, as well as immunomodulators.
Taking into account which part of the immune defense has been compromised, drugs such as interferon, echinacea herb, Taktivin, and so on can be prescribed.
When infected with bacteria, treatment of immunodeficiency states includes taking broad-spectrum antibacterial drugs in combination with immunoglobulins.
For viral diseases, the prescription of antiviral drugs (Valtrex, Acyclovir and a number of others) is indicated.
A diet (with an emphasis on protein foods), oxygen baths, and vitamin therapy are required. Physical activity is indicated. In some cases, a bone marrow transplant is performed.
There are medications that must be used if the immune system is weak. They are called immunomodulators. Perhaps the most famous and effective drug from this group is Transfer Factor.
This is a powerful new generation immunomodulator, which, when it enters the patient’s body, has the following effect:
- quickly restores the functioning of the immune system, normalizes metabolic processes;
- has a potentiating effect, enhancing the therapeutic effect of drugs taken together;
- blocks possible side effects from other drugs;
- “remembers” pathogenic microorganisms and, when they subsequently enter the body, gives a signal for their immediate destruction.
Transfer Factor is a 100% natural composition, so it has no side effects and virtually no contraindications.